
Concept explainers
Lactonization Methods
In Section we saw that hydroxy substituted
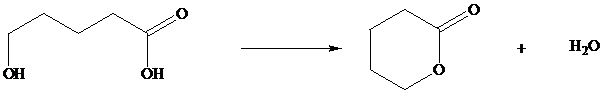
Many natural products are lactones, and chemists have directed substantial attention to developing alternative methods for their synthesis. The most successful of these efforts are based on electrophilic addition to the double bond of unsaturated carboxylic acids. For a generalized electrophilic reagent and, such reactions give a
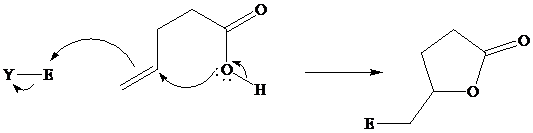
Although the curved arrows show the overall electron flow, the mechanism depends on the electrophilic reagent and normally involves more than one step.
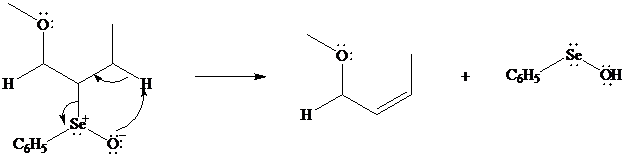
In iodolactonization the electrophilic atom, and represents a source of electrophilic iodine, usually or. In phenylselenolactonization, and is benzeneselenenyl chloride. Anti addition is observed in both iodo andphenylselenolactonization.
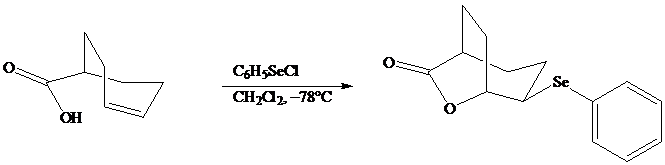
Both iodo andphenylselenolactonization offer the advantage of giving a product containing a
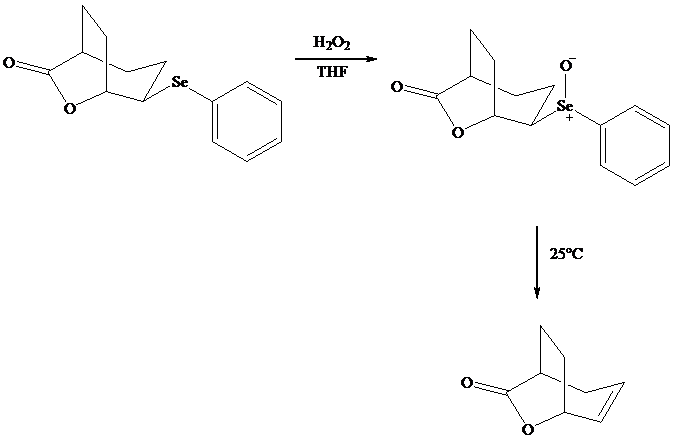
In eliminations of this type, H is always removed from the carbon to selenium that is remote from the lactone oxygen. Elimination is syn.
What is the structure of the
(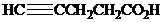 )? Anti addition to the triple bond occurs.
)? Anti addition to the triple bond occurs.
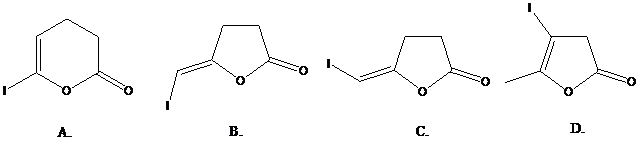
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry - Standalone book
- Q2) Write a reaction for the following, with explanation. part 1) Dimethyl ketone with Hydrogen cyanide (HCN) part2) Preparation of M. nitro chlorobenzene from benzene, using nitric acid Sulfuric acid, Chlorine, AICI, and any reagent you need. part3) Solvation of ethylene epoxide in acidic mediumarrow_forwardThe two reactions that follow involve nucleophilic substitution at an acyl carbon. For each reaction, show the electrophile and the nucleophile in the key bond- forming step, the corresponding tetrahedral addition intermediate, and the leaving group in the bond-breaking step. Write a general mechanistic scheme (use curved arrows to show bond-making and bond-breaking processes) for these reactions, keeping in mind that acid- and base-catalyzed processes will differ in their timing of proton transfers. 00 || || PhCOCPh+ C₂H5OH 00 |||| PhCOCPh+ 2 CH3NH H₂SO4 cat. O PhCOC₂H5+ PhCO₂H PhCNCHg + CH3NH PhCOarrow_forward1) How will you describe whether any compound has been oxidized or reduced? Support the answer with suitable examples. 2)Why carboxylic acid with a carbonyl group at 3rd position can be decarboxylated? 3) Explain why electrophilic aromatic substitution in Pyrrole takes place at C-2 positions whereas, in Pyridine it takes place at C-3 position? 4) List the following esters in order of decreasing reactivities towards hydrolysis with reason: Methyl benzoate, p-cyano methyl benzoate and p-hydroxy methyl benzoate 5)LDA is the base of choice for carbonyl compound to completely convert into enolate. Why?arrow_forward
- Concentrated phosphoric acid and concentrated sulfuric acid are common choices for dehydrating alcohols. Concentrated hydrochloric acid is not. What problem might occur if concentrated hydrochloric acid were substituted for phosphoric acid in the cyclohexene synthesis? allylic chlorination of the cyclohexene product may occur chlorocyclohexane may form as a byproduct the equilibrium will now favor formation of cyclohexanol by Le Chatelier's Principle hydrochloric acid is a weaker acid than phosphoric acidarrow_forward(b) Answer the following questions based on the compounds below. Jawab soalan berikut berdasarkan kepada sebatian di bawah. CI CI A в (i) Which compound has the higher boiling point? Explain. Sebatian manakah mempunyai takat didih yang lebih tinggi? Terangkan. (ii) Draw the SN2 mechanism for the reaction of compound A with sodium hydroxide, NaOH. Lukis mekanisma Sn2 bagi tindak balas antara sebatian A dengan natrium hidroksida, NaOH.arrow_forward(a) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Give two reasons.(b) How will you bring about the following converstions?(i) Propanone to propane (ii) Benzoyl chloride to benzaldehyde(iii) Ethanal to but-2-enalarrow_forward
- 3) Draw equations of the following reactions and and explain to which direction is the respective quillibrium shifted. a) cyclohexylamine + water b) aniline + sulphuric acid c) triethylamine + acetic acidarrow_forwardImagine that phenol (“hydroxybenzene”) and nitrobenzene are reacted (in separate beakers) with a hot solution containing both concentrated sulfuric acid and concentrated nitric acid. A) Phenol reacts much more quickly than benzene, and benzene reacts much more quickly that nitrobenzene. Explain this observation, using at least one appropriate reaction coordinate diagram as part of your answer (be sure to label your reaction diagram with appropriate structures). You do not need to include any complete mechanisms, but you may wish to use portions of mechanisms as part of your discussion.arrow_forwardSuggest syntheses for the following compound from the indicated starting materials and any other necessary inorganic or organic compounds.arrow_forward
- (b) Answer the following questions based on the compounds below. Jawab soalan berikut berdasarkan kepada sebatian di bawah. Br -Br A в (i) Which compound has the lower boiling point? Explain your answer. Sebatian manakah yang mempunyai takat didih yang lebih rendah? Terangkan jawapan anda. (ii) Draw the SN1 mechanism for the reaction of compound B with sodium hydroxide, NaOH. Lukiskan mekanisma SN1 bagi tindak balas antara sebatian B dengan natrium hidroksida, NaOH. (ii) Arrange the following compounds in increasing order of acidity. Susunkan sebatian berikut mengikut tertib menaik keasidannya. он HO, HO, Earrow_forwardHow is the mechanism for oxymercuration/demercuration reactions of alkenes understood? How are the (Markovnikov) products predicted and what reagents would you need to use to incorporate this reaction into a multistep synthesis strategy. Finally, what makes this a potentially more useful way to synthesize alcohols from alkenes than simple acid hydration?arrow_forwardIndustrial Chemistry, Benzene is a high-commodity aromatic material with many industrial applications but is produced in relatively small amounts through petroleum fractionation. Explain how Toluene can be converted to benzene, please be specific in terms of reagents and conditions.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

