
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.50SP
Show how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no more than four carbon atoms as your organic starting materials. Assume that para is the major product (and separable from ortho) in ortho, para mixtures.
- a. pentan-1-
amine - b. N-methylbutan-1-amine
- c. N-ethyl-N-propylbutan-2-amine
- d. N-benzylpropan-1-amine
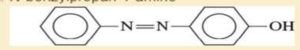
- e. 3-propylaniline
- f. 4-isobutylaniline
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Hh.10.
What is an acceptable
or preferred IUPAC
name for the following
compound?
a. (3R,4Z)-4-methyloct-4-en-3-amine
b. (3S,4Z)-4-methyloct-4-en-3-amine
c. (6R,4Z)-5-methyloct-4-en-6-amine
d. (6S,4Z)-5-methyloct-4-en-6-amine
e. (2R,4Z)-2-(1-aminopropyl)hex-2-
H2NI
ene
f. (2S,4Z)-2-(1-aminopropyl)hex-2-ene
Please explain the chosen letter.
Benzoic acid reacts with conc. HNO3 and conc.H2SO, to give
a. o-nitrobenzoic acid
b. p-nitrobenzoic acid
c. m-nitrobenzoic acid
d. p-dinitrobenzoic acid
Chapter 19 Solutions
Organic Chemistry (9th Edition)
Ch. 19.2A - Prob. 19.1PCh. 19.2B - Prob. 19.2PCh. 19.2B - Give correct names for the following amines:Ch. 19.3 - Prob. 19.4PCh. 19.4 - Prob. 19.5PCh. 19.6 - Rank each set of compounds in order of increasing...Ch. 19.8A - Prob. 19.7PCh. 19.8C - Prob. 19.8PCh. 19.8C - Prob. 19.9PCh. 19.8D - a. Show how fragmentation occurs to give the base...
Ch. 19.10B - Propose a mechanism for nitration of pyridine at...Ch. 19.10B - Prob. 19.12PCh. 19.10C - Prob. 19.13PCh. 19.10C - Prob. 19.14PCh. 19.11 - Propose a mechanism to show the individual...Ch. 19.11 - Prob. 19.16PCh. 19.12 - Give the products expected from the following...Ch. 19.13 - Prob. 19.18PCh. 19.13 - Prob. 19.19PCh. 19.14 - Prob. 19.20PCh. 19.15 - Prob. 19.21PCh. 19.15 - Prob. 19.22PCh. 19.16 - Prob. 19.23PCh. 19.17 - Prob. 19.24PCh. 19.17 - Prob. 19.25PCh. 19.18 - Prob. 19.26PCh. 19.19 - Prob. 19.27PCh. 19.20A - Addition of one equivalent of ammonia to...Ch. 19.20A - Prob. 19.29PCh. 19.20B - Show how you would accomplish the following...Ch. 19.20C - Prob. 19.31PCh. 19 - For each compound, 1. classify the...Ch. 19 - Prob. 19.33SPCh. 19 - Within each structure, rank the indicated...Ch. 19 - In each pair of compounds, select the stronger...Ch. 19 - Which of the following compounds are capable of...Ch. 19 - Complete the following proposed acid-base...Ch. 19 - Predict the products of the following reactions:...Ch. 19 - Prob. 19.39SPCh. 19 - Show how m-toluidine can be converted to the...Ch. 19 - The mass spectrum of tert-butylamine follows shows...Ch. 19 - Prob. 19.42SPCh. 19 - The following drugs are synthesized using the...Ch. 19 - Prob. 19.44SPCh. 19 - Synthesize from benzene. (Hint: All of these...Ch. 19 - Propose mechanisms for the following reactions.Ch. 19 - Prob. 19.47SPCh. 19 - Prob. 19.48SPCh. 19 - Prob. 19.49SPCh. 19 - Show how you can synthesize the following...Ch. 19 - Prob. 19.51SPCh. 19 - The alkaloid coniine has been isolated from...Ch. 19 - A chemist is summoned to an abandoned...Ch. 19 - Pyrrole undergoes electrophilic aromatic...Ch. 19 - Prob. 19.55SPCh. 19 - Prob. 19.56SPCh. 19 - An unknown compound shows a weak molecular ion at...Ch. 19 - A compound of formula C11H16N2 gives the IR,...Ch. 19 - (A true story.) A drug user responded to an ad...Ch. 19 - Prob. 19.60SPCh. 19 - Prob. 19.61SPCh. 19 - Prob. 19.62SPCh. 19 - Prob. 19.63SPCh. 19 - Prob. 19.64SPCh. 19 - Prob. 19.65SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can someone please help me with this question.arrow_forwardBe sure to answer all parts. An allylic alcohol contains an OH group on a carbon atom adjacent to a C-C double bond. Treatment of allylic alcohol A with HCl forms a mixture of two allylic chlorides, B and C. Select a stepwise mechanism that illustrates how both products are formed. HCT + H;O B. Part 1: Select the correct first step. :CI: :C: OH ä: HO. OH :CI: HO. Part 2 out of 4 Select all the appropriate resonance structures that result from the first step that is reproduced below. → resenance structures + H;O Next partarrow_forwardComplete the table provided below for nomenclature of Carboxylic Acid and Derivativesarrow_forward
- Write reactions of 2-methylbutanoic acid with the following reagents: a. CH3OH/H+ b. PBr5.arrow_forwardUse retrosynthetic analysis to show which reagents are necessary to carry out the following syntheses. a. b. d. NO2 O₂N OH Br NH2 CO₂H NH2arrow_forwardSuppose you have a mixture of these three compounds. Devise a chemical procedure based on their relative acidity or basicity to separate and isolate each in pure form.arrow_forward
- a. b. 1. Provide starting materials to synthesize the following amines by (a) Gabriel synthesis, (b) azide synthesis, or (c) reductive amination. 2. Write the structure for the major product for each of the following reactions. C. Br- 1. HNO3, H₂SO4 2. H₂, Pt 3. xs CH31 1. HNO3, H₂SO4 2. Br₂, FeBr3 3. H₂, Pt 4. NaNO2, HCI 5. CuBr NH₂ 1. NH₂ 2. NaOH, H₂O 3. NaBH3CN, [H*]arrow_forward3. Complete the following reactions. + ROH + phenylhydrazine `H Ph General Name: Ag(NH3)2OH + HCN H. General Name: + NABH4 + NaOHarrow_forward1. In the reactions involving the three isomeric alcohols with the formula C4H9OH, describewhat each of the following tests showed about reactivity of the -OH group and reactions of 1°,2°, and 3° alcohols.• the test with neutral KMnO4• the test with concentrated HCl2. Predict how the fourth alcohol with the formula C4H10O would react if tested with:• 0.01 M KMnO4• concentrated HCl at room temperatureExtend FurtherUse your observations of the solutions formed in the previous experiments and yourunderstanding of alcohols to complete a table like the one shown below. Research the meltingand boiling points to verify your answers.arrow_forward
- Consider the following alcohol, A. When reacted with one (or a combination of two) reagents in the "reagent box," A can be transformed into a variety of different molecules. H HO NaH: DMF POCI3 excess Nal DMSO SOBr₂ .Н PCI HO HO A Reagent Box 11 cat. H₂SO4 H₂O: acetone PBr3 Ho Hö List a reagent (or combination of two) from the reaction box capable of transforming A into the products given below. Note: there are many possible answers! Br POBr3 excess SOCI₂ НО TSCI CI OHarrow_forwardSuggest possible synthetic route for each of following transformations. Show the reagents, intermediates and products.arrow_forward1. Br₂, PBrg 2. H₂O H₂C OH H3C OH Br The a-bromination of carbonyl compounds by Br₂ in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br₂ reacts in an α- substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H3C :0: :0::Br: Br Br H3C CO-P H Br Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY