
Draw the structure for each of the following:
a. phenol
b. benzyl phenyl ether
c. benzonitrile
d. benzaldehyde
e. anisole
f. styrene
g. toluene
h. tert-buty lbenzene
i. benzyl chloride
a)
Interpretation:
The structure of phenol is to be drawn.
Concept introduction:
Phenols are defined as those compounds in which hydroxy group is attached directly to the benzene ring. Phenols and alcohols have so many similar properties.
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of phenol is shown below.
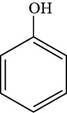
Figure 1
Explanation of Solution
The structure of phenol is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of phenol is shown below.
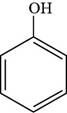
Figure 1
b)
Interpretation:
The structure of benzyl phenyl ether is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzyl phenyl ether is shown below.
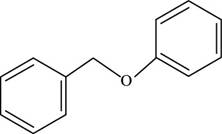
Figure 2
Explanation of Solution
The structure of benzyl phenyl ether is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl phenyl ether is shown below.

Figure 2
c)
Interpretation:
The structure of benzonitrile is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzonitrile is shown below.
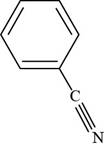
Figure 3
Explanation of Solution
The structure of benzonitrile is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzonitrile is shown below.
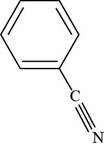
Figure 3
d)
Interpretation:
The structure of benzaldehyde is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzaldehyde is shown below.
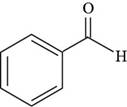
Figure 4
Explanation of Solution
The structure of benzaldehyde is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzaldehyde is shown below.
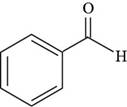
Figure 4
e)
Interpretation:
The structure of anisole is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of anisole is shown below.
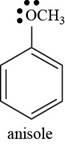
Figure 5
Explanation of Solution
The structure of anisole is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of anisole is shown below.
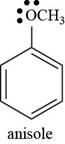
Figure 5
f)
Interpretation:
The structure of styrene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of styrene is shown below.
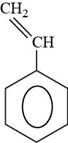
Figure 6
Explanation of Solution
The structure of styrene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of styrene is shown below.
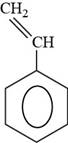
Figure 6
g)
Interpretation: The structure of toluene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of toluene is shown below.
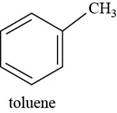
Figure 7
Explanation of Solution
The structure of toluene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of toluene is shown below.
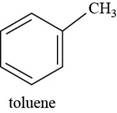
Figure 7
h)
Interpretation: The structure of tert-butyl benzene is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of tert-butyl benzene is shown below.
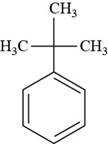
Figure 8
Explanation of Solution
The structure of tert-butyl benzene is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of tert-butyl benzene is shown below.

Figure 8
i)
Interpretation:
The structure of benzyl chloride is to be drawn.
Concept introduction:
The reactivity of benzene ring towards electrophilic aromatic substitution reactions is enhanced by the activating substituents. The deactivating substituents make the benzene ring less reactive. Some of the activating substituents are
Answer to Problem 42P
The structure of benzyl chloride is shown below.
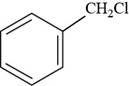
Figure 9
Explanation of Solution
The structure of benzyl chloride is drawn as follows.
The first step is to draw the structure of benzene ring. Then
Thus, the structure of benzyl chloride is shown below.

Figure 9
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- Don't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forward
- Show work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?arrow_forward13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. cleavage Bond A •CH3 + 26.← Cleavage 2°C. + Bond C +3°C• CH3 2C Cleavage E 2°C. 26. weakest bond Intact molecule Strongest 3°C 20. Gund Largest argest a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. C Weakest bond A Produces Most Bond Strongest Bond Strongest Gund produces least stable radicals Weakest Stable radical b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 13°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. • CH3 methyl radical Formed in Gund A Cleavage c.…arrow_forwardBr. COOH Br, FCH COOH E FeBr ASOCI B NH (CH,CO),OD Br₂ 2 C alcKOHarrow_forward
- Find A to F (all)arrow_forwardShow work. don't give Ai generated solutionarrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. < cleavage Bond A • CH3 + 26. t cleavage 2°C• +3°C• Bond C Cleavage CH3 ZC '2°C. 26. E Strongest 3°C. 2C. Gund Largest BDE weakest bond In that molecule a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest C bond Produces A Weakest Bond Most Strongest Bond Stable radical Strongest Gund produces least stable radicals b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 人 8°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. methyl radical •CH3 formed in bund A Cleavagearrow_forwardWhich carbocation is more stable?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
