
(a)
Interpretation:
The structure of
Concept introduction:
Answer to Problem 16.2P
The structure of
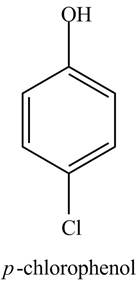
Explanation of Solution
The aromatic compound,

Figure 1
The structure of
(b)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
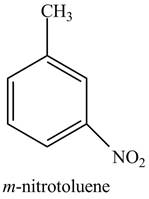
Explanation of Solution
The aromatic compound,
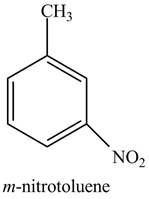
Figure 2
The structure of
(c)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
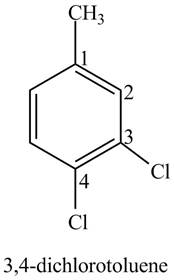
Explanation of Solution
The aromatic compound,

Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
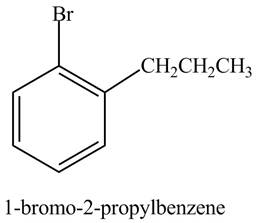
Explanation of Solution
The aromatic compound,
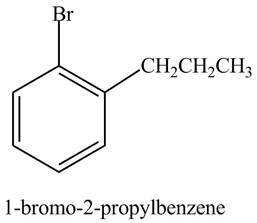
Figure 4
The structure of
(e)
Interpretation:
The structure of methyl phenyl ether is to be stated.
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of methyl phenyl ether is shown below.
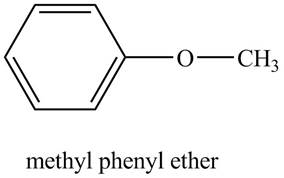
Explanation of Solution
The compound, methyl phenyl ether contains a methyl group and a phenyl ring as a substituent attached to the oxygen atom of the ether functional group position as shown below.

Figure 5
The structure of methyl phenyl ether is shown in Figure 5.
(f)
Interpretation:
The structure of benzyl methyl ether is to be stated.
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of benzyl methyl ether is shown below.
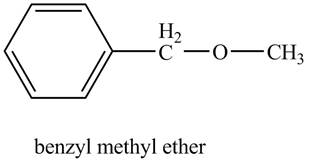
Explanation of Solution
Benzene ring with a methyl substituent is known as a benzyl group. The compound, benzyl methyl ether contains a methyl group and a benzyl group as a substituent on the oxygen atom of the ether functional group as shown below.
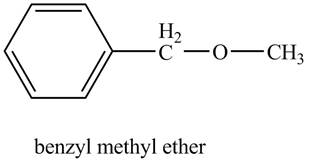
Figure 6
The structure of benzyl methyl ether is shown in Figure 6.
(g)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
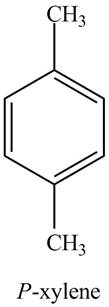
Explanation of Solution
The common name of

Figure 7
The structure of
(h)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
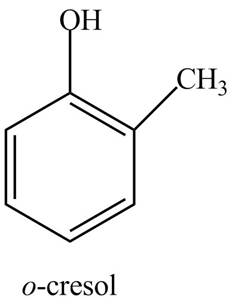
Explanation of Solution
The common name of
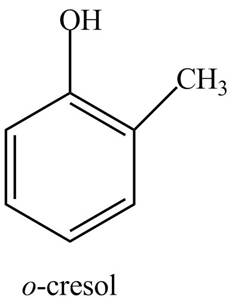
Figure 8
The structure of
(i)
Interpretation:
The structure of
Concept introduction:
Aromatic compounds are those compounds which contains at least one aromatic ring. The naming of aromatic compounds uses the same rules as aliphatic compounds. The six-membered aromatic ring is named as benzene. IUPAC stands for International Union of Pure and Applied Chemistry. The IUPAC gave certain rules which are used worldwide to name the organic compounds. Due to IUPAC naming, each compound has a unique name which is different from their common name.
Answer to Problem 16.2P
The structure of
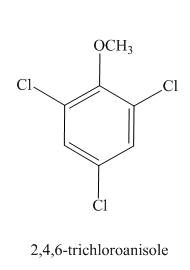
Explanation of Solution
The aromatic compound,

Figure 9
The structure of
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry
- Compound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forwardCompound A, C3H7Br, does not react with cold dilute potassium permanganate solution. Upon treatment with potassium hydroxide in ethanol, A gives only product B, C3H6. Unlike A, B decolourises potassium permanganate solution. Ozonolysis of Bgives C, C2H4O, and D, CH2O. Suggest the structural formulae of A, B, C and D.Write the equations for all the reactions involved.arrow_forwardProvide the structure of the major organic product of the following reaction and? explain the stereochemistry which results in this product. 2-Pentanol reacting with 1.) PBr3, pyridine 2.) NaCNarrow_forward
- On being heated with a solution of sodium ethoxide in ethanol, compound A (C7H15Br) produced a mixture of two alkenes B and C, each of which had the molecular formula C7H14. Catalytic hydrogenation of major isomer B or minor isomer C gave only 3-ethylpentane. Suggest structures and mechanisms for compounds A, B, and C consistent with these observations.arrow_forwardGive the series of reactions below, identify and give the iupac name for the following compounds, in short identify and name A,B,Carrow_forwardGive the structure of compound 1 and compound 2arrow_forward
- Deduce the structure of compound C.arrow_forwardDraw/outline a reasonable and detailed mechanism for the dehydration of 2-methylcyclohexanol catalyzed by phosphoric acid. Use curved arrows to show the flow of electrons and draw the structures of all intermediates and byproducts formed in the reaction. Predict and rationalize the expected product distribution based on thermodynamic aspectsarrow_forwardCompound A produce compound D while undergo Friedel Crafts Alkylation. Compound D is then oxidized and produce compound E (C11H12O3) as a major product.What are the possible structural formula of compound D and E?arrow_forward
- give the structural formula for compounds A to Garrow_forwardPlease propose a structural formula for compound A, C4H10O, consistent with the following 1H-NMR and IR spectra. Assign all the appropriate peaks in the IR and NMR spectra and provide a short narrative describing what structural information each piece of data provided.arrow_forwardThe following equation shows the bromination of compound 1. Propose a structure for product D and a curved arrow mechanism that accounts for its formation featuring the initiation, propagation and termination steps. can you also discuss the stereochemical outcome.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
