
Concept explainers
(a)
Interpretation:
The following pair of molecules should be classified as stereoisomers, constitutional isomers, or identical molecules:

Concept Introduction:
Isomers are the compounds having same molecular formula but different structure. The phenomenon possessed by the molecules is known as isomerism.
Compounds having same molecular formula but possessing different connectivity of atoms or group of atoms are known as constitutional isomers.
The compounds which have same molecular formula, same connectivity of bond but the arrangement of atoms in space is different are known as stereoisomers.
The compounds having same bond connectivity and same molecular formula are known as identical molecules.
(b)
Interpretation:
The following pair of molecule should be classified as stereoisomers, constitutional isomers, or identical molecules:
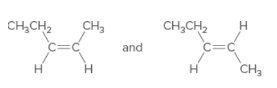
Concept Introduction:
Isomers are the compounds having the same molecular formula but different structures. The phenomenon possessed by the molecules is known as isomerism.
Compounds having same molecular formula but possessing different connectivity of atoms are known as constitutional isomers.
The compounds which have the same molecular formula, same connectivity of bond but the arrangement of atoms in space is different are known as stereoisomers.
The compounds having the same bond connectivity and same molecular formula are known as identical molecules.
(c)
Interpretation:
The following pair of molecule should be classified as stereoisomers, constitutional isomers, or identical molecules:

Concept Introduction:
Isomers are the compounds having same molecular formula but different structures. The phenomenon possessed by the molecules is known as isomerism.
Compounds having same molecular formula but possessing different connectivity of atoms are known as constitutional isomers.
The compounds which have the same molecular formula, same connectivity of bond but the arrangement of atoms in space is different are known as stereoisomers.
The compounds having the same bond connectivity and same molecular formula are known as identical molecules.
(d)
Interpretation:
The following pair of molecule should be classified as stereoisomers, constitutional isomers, or identical molecules:

Concept Introduction:
Isomers are the compounds having the same molecular formula but different structure. The phenomenon possessed by the molecules is known as isomerism.
Compounds having same molecular formula but possessing different connectivity of atoms are known as constitutional isomers.
The compounds which have the same molecular formula, same connectivity of bond but the arrangement of atoms in space is different are known as stereoisomers.
The compounds having the same bond connectivity and same molecular formula are known as identical molecules.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
General, Organic, & Biological Chemistry
- Don't USE AIarrow_forwardShow the full mechanism of how the molecule ((1E, 3E, 5E)-1-methoxyhepta-1,3,5-triene) is built using substitution and elimination reactions. You can start with an alkane of any carbon length with any number of leaving groups attached or with a alkoxide of any carbon length (conjugate base of an alcohol). Show each step and and explanation for each reaction. Also include why the reagents and solvents were picked and what other products can be expected.arrow_forwardProblems 1. Acids (11) and (12) were both made by Grignard addition to CO2 rather than by cyanide displacement (p T 80). Why? (11) -CO2H MeO- (12) CO,Harrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





