
Concept explainers
(a)
Interpretation:
The relationship between the given pair of molecules needs to be explained.

Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
When a molecule has more than one chiral center, another class of stereoisomers can be defined: Diastereomers, are the stereoisomers, which are not mirror images of each other.
(b)
Interpretation:
The relationship between the given pair of molecules needs to be explained.
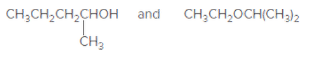
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
When a molecule has more than one chiral center, another class of stereoisomers can be defined: Diastereomers, are the stereoisomers, which are not mirror images of each other.
(c)
Interpretation:
The relationship between the given pair of molecules needs to be explained.

Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
When a molecule has more than one chiral center, another class of stereoisomers can be defined: Diastereomers, are the stereoisomers, which are not mirror images of each other.
(d)
Interpretation:
The relationship between the given pair of molecules needs to be explained.
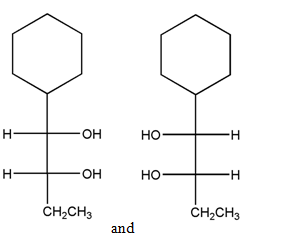
Concept Introduction:
Identical molecules are the ones with no isomers, neither constitutional isomers nor stereoisomers. Identical molecules have the same structural arrangement of atoms and the same three-dimensional arrangement.
Isomers are the molecules with the same formula but either with different structural connectivity (constitutional isomers) or different three-dimensional arrangement (stereoisomers).
A tetrahedral carbon atom bonded to four different groups is called a chiral center. A Molecule having at least one chiral center is a chiral molecule. Molecules that do not have any chiral centers are called achiral. Identical molecules do not have any chiral centers; therefore, they are achiral.
When the mirror images of a chiral molecule are not superimposable, those mirror images become stereoisomers called enantiomers.
When a molecule has more than one chiral center, another class of stereoisomers can be defined: Diastereomers, are the stereoisomers, which are not mirror images of each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
General, Organic, & Biological Chemistry
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




