
Concept explainers
(a)
Interpretation:
The picture of the solution made by mixing the solutions A and B together after the precipitation reaction takes place is to be drawn.
Concept Introduction:
The molarity is the number of moles of the solute dissolved per liter volume of the solution. It is represented in mathematical term such that,
Where, n is the number of moles,
V is the volume of the solution
The limiting regent is that reactant of the reaction which control the amount of the product formed.
(a)
Answer to Problem 63A
The diagram shows the relative volume compared to the both solutions
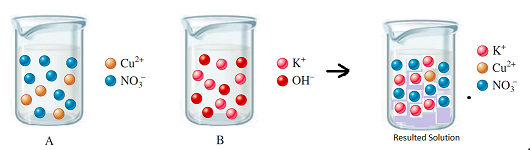
Explanation of Solution
Given information:
The volume and molarity of the copper nitrate solution are
And,
The volume and molarity of the B solution is,
Calculation:
Where, n is the number of moles,
V is the volume of the solution
The number of moles of the solution A is,
Similarly, the number of moles of solution B is,
The balanced chemical rection between the solution A and solution B is,
The number of moles of calcium hydroxide formed from the calcium nitrate is,
And from the potassium hydroxide is,
So, potassium hydroxide is the limiting reagent.
The diagram shows the relative volume compared to the both solutions
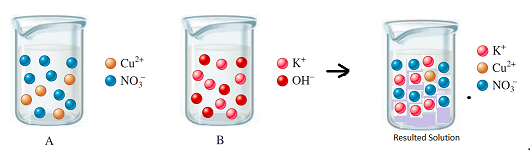
Thus, the diagram shows the relative volume of the given solutions.
(b)
Interpretation:
The concentration of the ions and mass of solid is to be determined.
Concept Introduction:
The molarity is the number of moles of the solute dissolved per liter volume of the solution. It is represented in mathematical term such that,
(b)
Answer to Problem 63A
The concentration of all ions left in the solution are
Explanation of Solution
Given information:
The volume and molarity of the copper nitrate solution are
And,
The volume and molarity of the B solution is,
Calculation:
Where, n is the number of moles,
V is the volume of the solution
The mass of the copper hydroxide is calculated as,
The initial moles of
Similarly, used moles of copper ion is 3 moles. So, the remaining number of moles is,
Total volume is 4.00 L
The concertation of remaining copper ion is,
Similarly, the number of moles of hydroxide ion is,
The used number of moles of hydroxide ion is,
So, the remaining number of moles is zero of hydroxide ions.
The remaining number of moles of nitrate ions is,
The remaining mole of potassium ion is,
The concentration of nitrate and potassium ions is,
And,
Thus, the concentration of all ions left in the solution are
Chapter 15 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





