
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 58P
Zolpidem (trade name Ambien) promotes the rapid onset of sleep, making it a widely prescribed drug for treating insomnia.
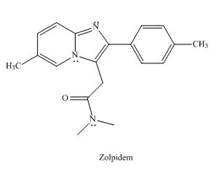
a In what type of orbital does the lone pair on each N atom in the heterocycle reside?
b. Explain why the bicyclic ring system that contains both N atoms is
c. Draw all reasonable resonance structures for the bicyclic ring system.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Zolpidem (trade name Ambien) promotes the rapid onset of sleep, making it a widely prescribed drug for treating insomnia.
a.In what type of orbital does the lone pair on each N atom in the heterocycle reside?
b. Explain why the bicyclic ring system that contains both N atoms is aromatic.
c.Draw all reasonable resonance structures for the bicyclic ring system.
The purine heterocycle occurs commonly in the structure of DNA.
a. How is each N atom hybridized?
b. In what type of orbital does each lone pair on a N atom reside?
c. How many a electrons does purine contain?
d. Why is purine aromatic?
purine
INSTRUCTIONS: Choose the letter of the BEST answer for each item.
1. How many lone pairs are involved in sustaining the conjugation of pyridine?
A. One
B. Two
C. Three
D. Four
2. Benzopyrene, naphthalene and pyrene are members of these group of aromatic compounds:
A. Benzenoid aromatic compounds
B. Non-benzenoid aromatic compounds
C. Heterocyclic aromatic compounds
D. Heteronuclear compounds
3. What type of aromatic compound is pyridine?
A. Heterocyclic aromatic compound
B. Benzenoid aromatic compound
C. Non-benzenoid aromatic compound
D. Homonuclear cyclic compound
4. What property of aromatic rings prevent the involvement of the conjugated structure to addition reactions?
A. Radical stabilization
B. Resonance stability
C. Inductive effect
D. Aromatic effect
5. Which electrophilic aromatic substitution reaction is described when aniline is transformed into para-aminotolouene?
A. Nitration
B. Friedel Crafts alkylation
C. Oxidation
D. Halogenation
6. What type of relationship does…
Chapter 15 Solutions
Organic Chemistry (6th Edition)
Ch. 15.2 - Prob. 1PCh. 15.2 - Problem 17.2 What orbitals are used to form the...Ch. 15.3 - Prob. 4PCh. 15.3 - Problem-17.5 What is the structure of propofol,...Ch. 15.6 - Prob. 7PCh. 15.8 - Prob. 8PCh. 15.8 - Prob. 11PCh. 15.8 - Prob. 12PCh. 15.8 - Problem 17.16 Rank the following compounds in...Ch. 15.8 - Problem 17.17 Draw the seven resonance structures...
Ch. 15 - 17.23 Name each compound and state how many lines...Ch. 15 - Prob. 21PCh. 15 - Prob. 22PCh. 15 - 17.27 Give the IUPAC name for each compounds.
a....Ch. 15 - 17.28 Draw a structure corresponding to each...Ch. 15 - 17.29 a. Draw the 14 constitutional isomers of...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - 17.38
How many electrons does C contain?
How...Ch. 15 - Prob. 36PCh. 15 - 17.40 Explain the observed rate of reactivity of...Ch. 15 - 17.41 Draw a stepwise mechanism for the following...Ch. 15 - Prob. 39PCh. 15 - 17.43 Draw additional resonance structures for...Ch. 15 - Prob. 41PCh. 15 - Prob. 42PCh. 15 - 17.46 Which compound in each pair is the stronger...Ch. 15 - 17.47 Treatment of indene with forms its...Ch. 15 - Prob. 45PCh. 15 - 17.49 Draw the conjugate bases of pyrrole and...Ch. 15 - 17.50 a. Explain why protonation of pyrrole occurs...Ch. 15 - Prob. 48PCh. 15 - Prob. 49PCh. 15 - 17.53 How many signals does each compound...Ch. 15 - 17.54 Which of the diethylbenzene isomers (ortho,...Ch. 15 - 17.55 Propose a structure consistent with each...Ch. 15 - 17.56 Propose a structure consistent with each...Ch. 15 - 17.57 Thymol (molecular formula ) is the major...Ch. 15 - 17.58 You have a sample of a compound of molecular...Ch. 15 - 17.59 Explain why tetrahydrofuran has a higher...Ch. 15 - 17.60 Rizatriptan (trade name Maxalt) is a...Ch. 15 - 17.61 Zolpidem (trade name Ambien) promotes the...Ch. 15 - 17.62 Answer the following questions about...Ch. 15 - 17.63 Stanozolol is an anabolic steroid that...Ch. 15 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a.How many π electrons does C contain? b.How many π electrons are delocalized in the ring? c.Explain why C is aromatic.arrow_forwardC. D. O: :O: The lone pair in compound C is Compound C is In compound D, not aromatic. aromatic. delocalized. not delocalized. one lone pair is delocalized. both lone pairs are not delocalized. both lone pairs are delocalized. Compoundarrow_forwardStanozolol is an anabolic steroid that promotes muscle growth. Although stanozolol has been used by athletes and body builders, many physical and psychological problems result from prolonged use and it is banned in competitive sports. a. Explain why the nitrogen heterocycle—a pyrazole ring—is aromatic. b. In what type of orbital is the lone pair on each N atom contained? c. Draw all reasonable resonance structures for stanozolol. d. Explain why the pKa of the N – H bond in the pyrazole ring is comparable to the pKa of the O–H bond, making it considerably more acidic than amines such as CH3NH2 (pKa = 40).arrow_forward
- Stanozolol is an anabolic steroid that promotes muscle growth. Although stanozolol has been used by athletes and body builders, many physical and psychological problems result from prolonged use and it is banned in competitive sports. a.Explain why the nitrogen heterocycle—a pyrazole ring—is aromatic. b.In what type of orbital is the lone pair on each N atom contained? c. Draw all reasonable resonance structures for stanozolol. d.Explain why the pKa of the N—H bond in the pyrazole ring is comparable to the pKa of the O—H bond, making it considerably more acidic than amines such as CH3NH2 (pKa = 40).arrow_forwardWhich (if any) lone pairs are participating in aromaticity?arrow_forwardplease explain why they are aromatic.?arrow_forward
- a. Identify the functional groups in the ball-and-stick model of neral, a compound with a lemony odor isolated from lemon grass. b. Draw a skeletal structure of a constitutional isomer of neral that should be more water soluble. c. Label the most electrophilic carbon atom.arrow_forwardwhich compound is not Aromatic H BJ N. c) D) A) B. HBarrow_forwardThe aromatic energy diagram below corresponds to an anti-aromatic compound.?arrow_forward
- Which structure is aromatic and has six electrons In the conjugated system? Click on a letter A through D to answer. H A. C. D. В.arrow_forward2. Which structure is NOT aromatic? a d. H3C :Br: DOarrow_forwardLabel each compound as aromatic, antiaromatic, or not aromatic. Assume all completely conjugated rings are planar. Å a. b. C. d.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY