
Concept explainers
(a)
Interpretation:
The inorganic product formed when butanal undergoes Tollen’s test has to be given.
Concept Introduction:
In
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
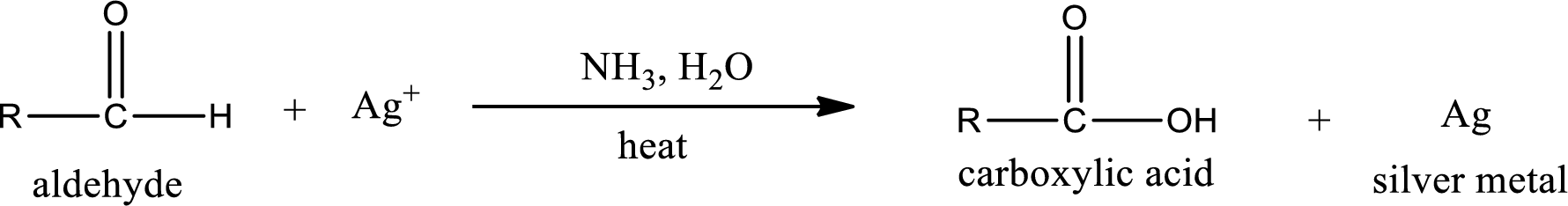
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
(a)
Answer to Problem 15.79EP
The inorganic product formed is silver metal.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
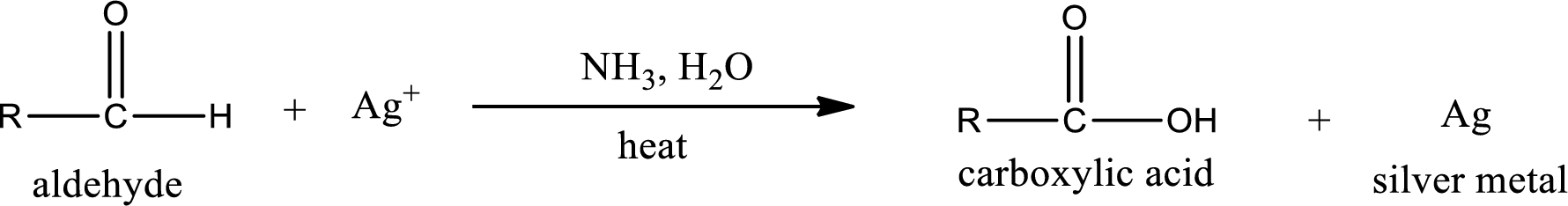
Given aldehyde is butanal and the structure can be given as shown below,
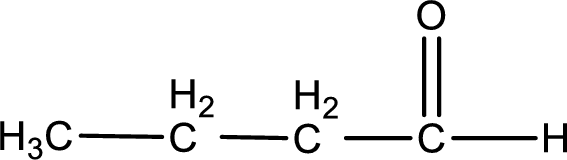
This on reaction with Tollen’s reagent gives carboxylic acid and silver metal as the product. The structure of the inorganic product formed and the complete reaction can be given as shown below,
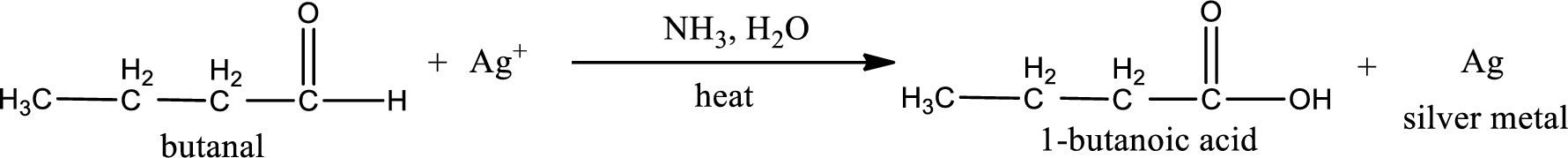
Silver metal is formed as the inorganic product when butanal undergoes Tollen’s test.
The inorganic product formed is given.
(b)
Interpretation:
The inorganic product formed when 2-butanone undergoes Tollen’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
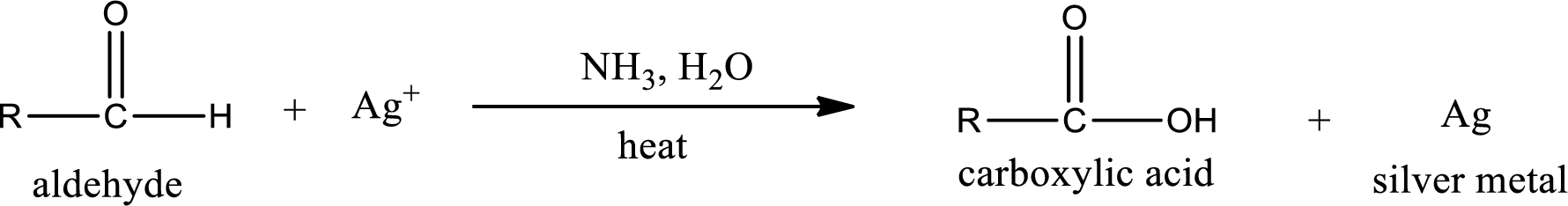
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
(b)
Answer to Problem 15.79EP
No inorganic product is obtained as 2-butanone does not undergo Tollen’s test.
Explanation of Solution
Aldehydes undergo Tollen’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
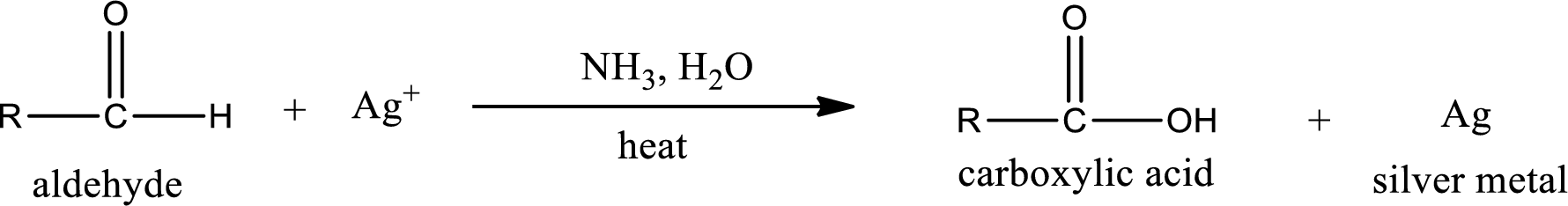
Given compound is a ketone that is 2-butanone and the structure can be given as shown below,
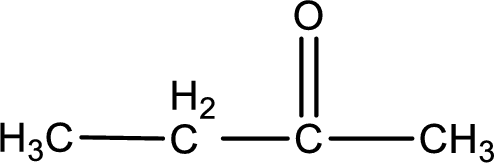
This on reaction with Tollen’s reagent does not give oxidized product. Therefore, no reaction takes place when 2-butanone reacts with Tollen’s reagent.
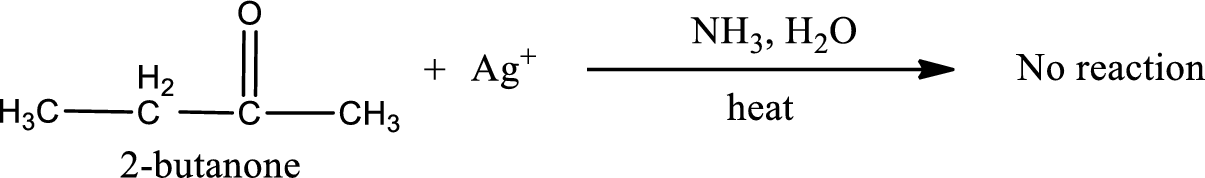
No inorganic product is formed when 2-butanone undergoes Tollen’s test.
No reaction takes place when 2-butanone undergoes Tollen’s test.
(c)
Interpretation:
The inorganic product formed when 3-methylpentanal undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
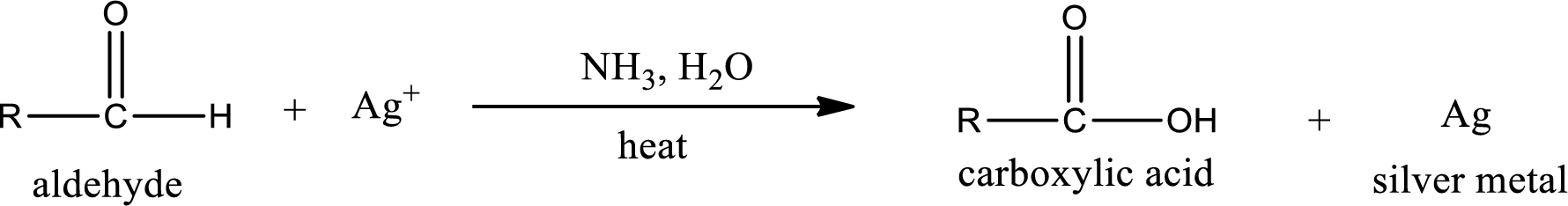
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
(c)
Answer to Problem 15.79EP
The inorganic product formed is
Explanation of Solution
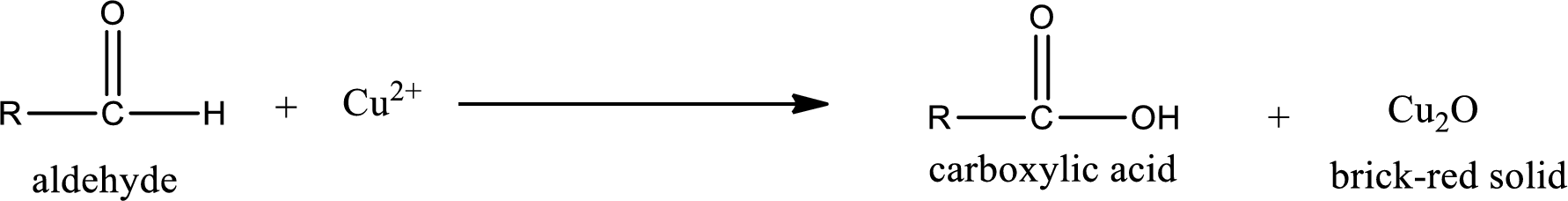
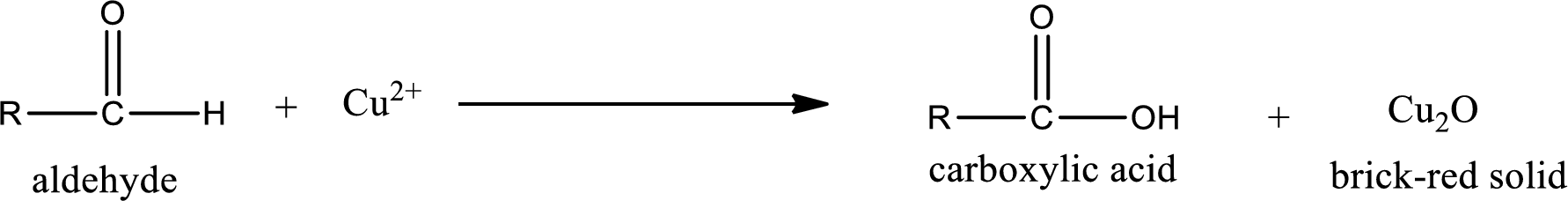
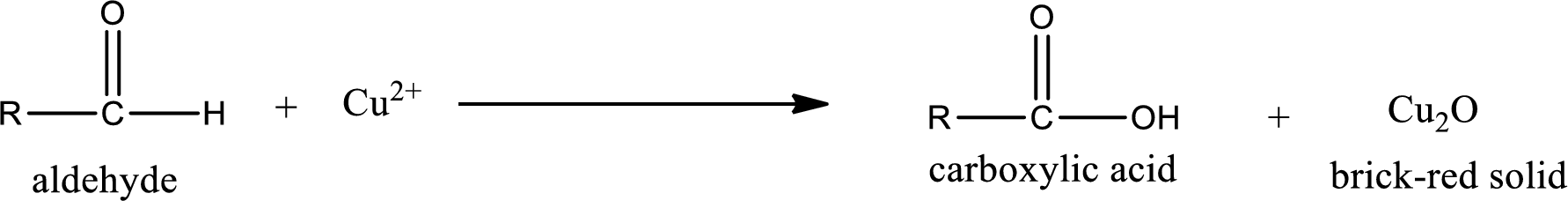
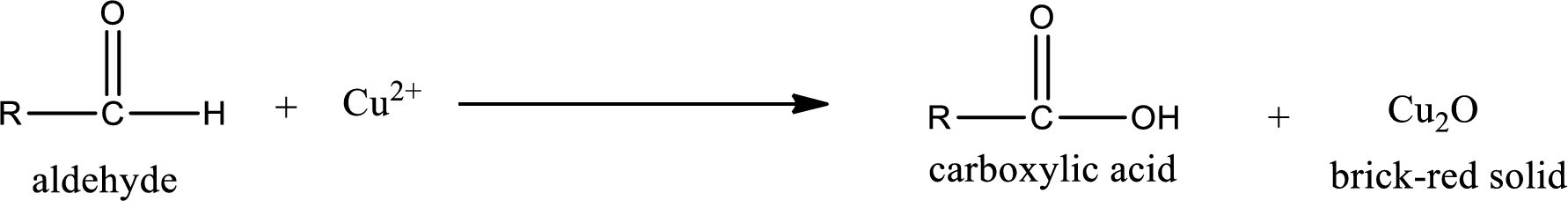
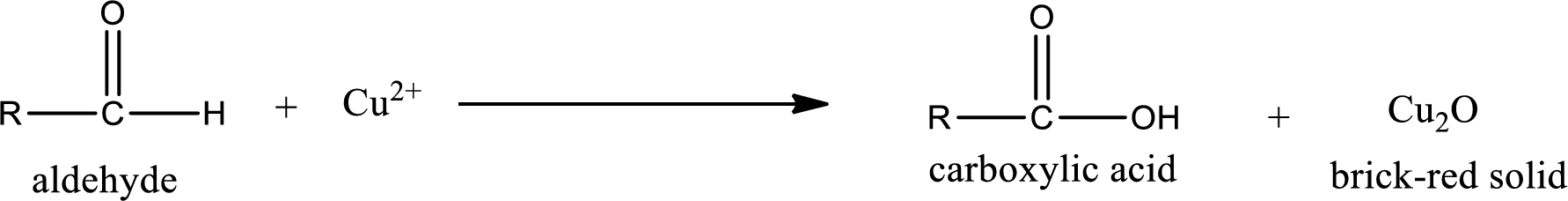
Aldehydes undergo Benedicts’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
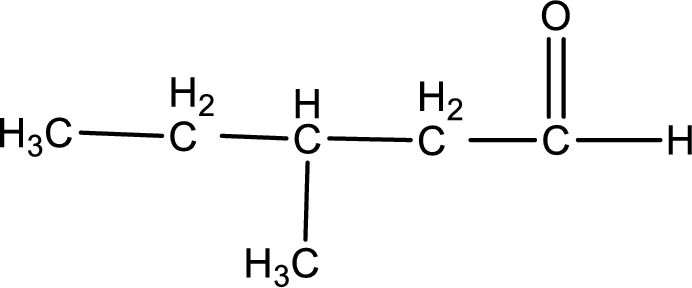
Given aldehyde is 3-methylpentanal and the structure can be given as shown below,
This on reaction with Tollen’s reagent gives carboxylic acid and Copper(I) oxide as the product. The inorganic product formed and the complete reaction can be given as shown below,
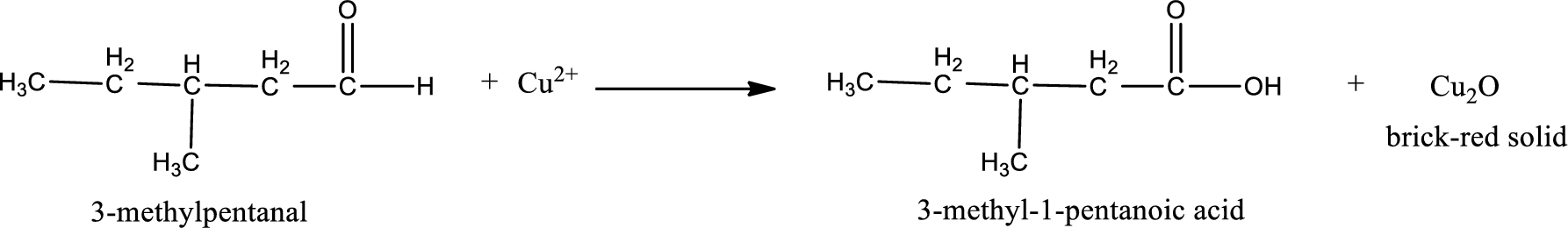
The inorganic product formed when 3-methylpropanal undergoes Benedict’s test is given.
(d)
Interpretation:
The inorganic product formed when 3-pentanone undergoes Benedict’s test has to be given.
Concept Introduction:
In organic chemistry, oxidation reaction is referred to the number
In organic chemistry, reduction reaction is referred to the number
Alcohols undergo oxidation reaction and reduction reaction. This depends upon the number of hydrogen atoms that is bonded to the alpha carbon atom. Primary and secondary alcohol undergoes oxidation reaction while tertiary alcohol does not undergo oxidation reaction. Primary alcohols undergo oxidation to give aldehyde and carboxylic acid as product. Secondary alcohol undergoes oxidation to give ketone as the product.
Aldehyde undergoes oxidation to give carboxylic acid as the product while ketone does not undergo oxidation reaction.
Tollen’s test:
This is also known as silver mirror test. The reagent that is used in Tollen’s test is silver nitrate and ammonia in water. Aldehyde reacts with Tollen’s reagent, where the silver ion is reduced to silver metal and the aldehyde is oxidized to carboxylic acid.
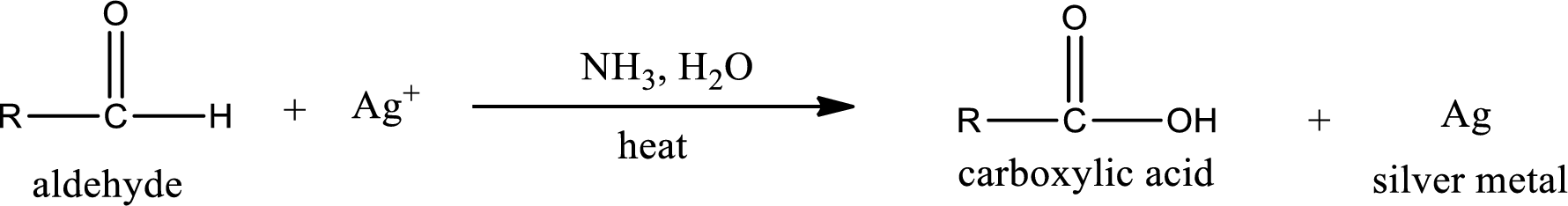
Ketone does not undergo Tollen’s test to deposit silver metal.
Benedict’s test:
This test is also similar to Tollen’s test. In this test,
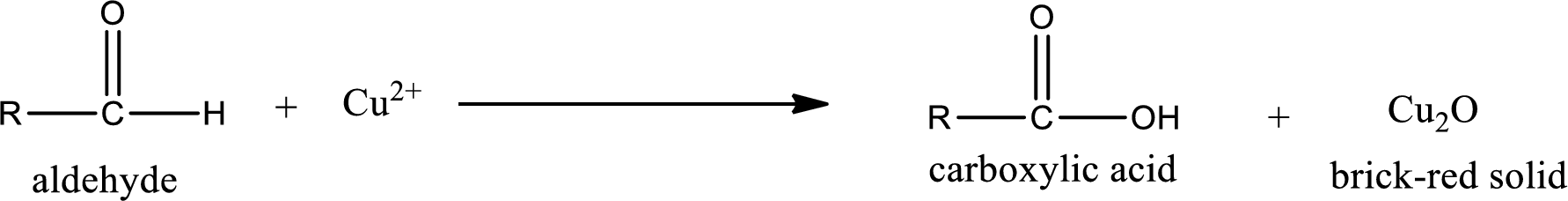
(d)
Answer to Problem 15.79EP
No inorganic product is formed when 3-pentanone undergoes Benedict’s test.
Explanation of Solution
Aldehydes undergo Benedict’s test. The product formed when aldehyde undergo oxidation is a carboxylic acid. The general oxidation reaction for aldehyde can be given as,
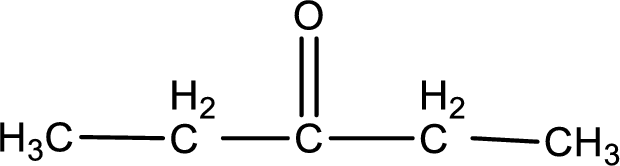
Given compound is a ketone. The name of ketone is 3-pentanone and the structure can be given as shown below,
This on reaction with Benedict’s reagent does not give oxidized product. Therefore, no reaction takes place when 3-pentanone undergoes Benedict’s test.
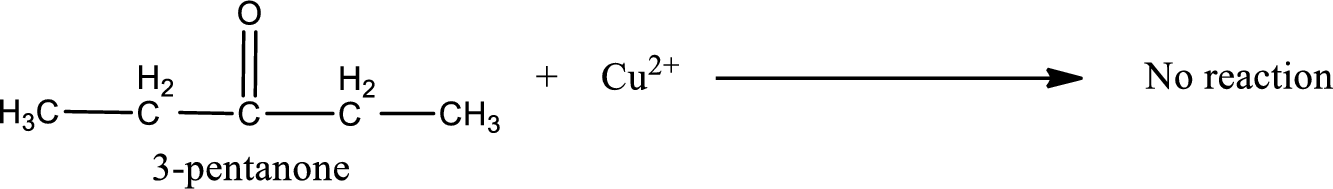
No inorganic product is formed when 3-pentanone undergo Benedict’s test.
No reaction takes place when 3-pentanone undergoes Benedict’s test.
Want to see more full solutions like this?
Chapter 15 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- 17-18 Draw structural formulas for these ketones. (a) Ethyl isopropyl ketone (b) 2-Chiorocyclohexanone (c) 2,4-Dimethyl-3-pentanone (d) Diisopropyl ketone (e) Acetone (f) 2,5-Dimethylcyclohexanonearrow_forward14-51 (Chemical Connections 14B) When was nitroglycerin discovered? Is this substance a solid, a liquid, or a gas'arrow_forward17-67 Draw structural formulas for these compounds. (a) 1-Chloro-2-propanone (b) 3-Hydroxybutanal (c) 4-Hydroxy-4-methyl-2-pentanone (d) 3-Methyl-3-phenylbutanal (e) 1,3-Cyclohexanedione (f) 5-Hydroxyhexanalarrow_forward
- 16-24 What is the common name for each of the following unbranched carboxylic acids? a. four-carbon monocarboxylic acid b. three-carbon monocarboxylic acid c. four-carbon dicarboxylic acid d. three-carbon dicarboxylic acidarrow_forwardFor embalming purposes, phenol has the properties of preserving, disinfecting, and ___ . cauterizing cut tissues adding a pink glow to the body adding a nice fragrance anticoagulantarrow_forward16-32 Each of the following acids contains an additional type of functional group besides the carboxyl group. For each acid, specify the noncarboxyl functional group present. a. Fumaric acid c. Malic acid b. Pyruvic acid d. Tartaric acid 1633 Cive the IUPAC name for each of the acids in Problem 16-31. Coutu 16-34 Give the IUPAC name for each of the acids in Problem 16-32.arrow_forward
- 16-48 How many acidic hydrogen atoms are present in each of the following carboxylic acids? a. Acetic acid c. Propanoic acid b. Benzoic acid d. Glutaric acid Whet is the charge on t1arrow_forwardWhat is the name of the major product formed during the reaction between berzoyl chloride and phenol? a. phenyl benzoate b. benzyl ester C. cyclopentanoate d. benzyl phenoate e. benzenecarboxylic acid O a O b O c earrow_forwardWhat chemical test can differentiate these two organic compounds? OH ОН and Tollens' test Lucas Test Phenol Test O 2,4-DNPH testarrow_forward
- 16-16 Assign an IUPAC name to each of the following car- 16 Assign an IUPAC name to each of the following car- boxylic acids a. HO, b. HO d. СООН HO, C.arrow_forward1. Draw the carboxylic acids formed by the oxidation of each of the following molecules. HOH₂C H2 CH3 H3CH2C H + 2. Draw the esters formed by the following reactions. a. H3C H3C-C-C H₂ H3C. OH H3C OH b. H3C H3CHCH2CH2C- རི OH + H3C. `CH2CH3 OH H₂ 3. Indicate the products of the following reaction: LCH CH3 + H₂O H3CH2CH2C CH₂OHarrow_forwardChemical Properties of Aldehydes and Ketones Samples Tollens Iodoform Chromic Acid Fehling’s Ethanol Butanol 2-propanol 2-methanol-2-propanol Phenolarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





