
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.8, Problem 14P
Use resonance theory to explain why the labeled C – O bond lengths are equal in the acetate anion.
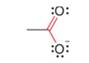
acetate
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the energy diagram for the reaction of the acetate ion with methyl bromide (CH3Br). Is the reaction endothermic or exothermic?
Write the structural formulas of trichloroacetic acid and acetic acid and explain why trichloroacetic acid is the much stronger acid of the two.
Describe a simple physical test that would serve to distinguish between propionic acid
and ethyl acetate.
O words
ņ
画
Chapter 14 Solutions
Organic Chemistry (6th Edition)
Ch. 14.1 - Prob. 2PCh. 14.2 - Problem 16.3 Draw a second resonance structure for...Ch. 14.2 - Prob. 4PCh. 14.2 - Problem 16.5 Farnesyl diphosphate is synthesized...Ch. 14.3 - Prob. 6PCh. 14.4 - Prob. 7PCh. 14.4 - Prob. 8PCh. 14.8 - Problem 16.12 Using hybridization, predict how the...Ch. 14.8 - Problem 16.13 Use resonance theory to explain why...Ch. 14.9 - Prob. 15P
Ch. 14.9 - Prob. 16PCh. 14.10 - Problem 16.17 Draw a stepwise mechanism for the...Ch. 14.11 - Prob. 19PCh. 14.12 - Problem 16.19 Draw the product formed when each...Ch. 14 - Prob. 32PCh. 14 - 16.31 Which of the following systems are...Ch. 14 - 16.32 Draw all reasonable resonance structures for...Ch. 14 - Prob. 35PCh. 14 - 16.35 Explain why the cyclopentadienide anion A...Ch. 14 - Prob. 38PCh. 14 - 16.37 Draw the structure of each compound.
a. in...Ch. 14 - 16.41 Draw the products formed when each compound...Ch. 14 - Prob. 44PCh. 14 - 16.43 Treatment of alkenes A and B with gives the...Ch. 14 - 16.44 Draw a stepwise mechanism for the following...Ch. 14 - Prob. 47PCh. 14 - 16.57 A transannular Diels–Alder reaction is an...Ch. 14 - Draw a stepwise mechanism for the following...Ch. 14 - Prob. 62PCh. 14 - Prob. 63PCh. 14 - Prob. 64PCh. 14 - Prob. 65PCh. 14 - 16.65 The treatment of isoprene with one...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the full reaction. ısı=heatarrow_forwardWrite the two-resonance structures for the acetate ion.arrow_forwardFormation of NO from nitrogen and oxygen is an endothermic process. Which statements are true about the bonds of this reaction? a.) Products have lower PE because product bonds are stronger than reactants b.) Products have higher PE because product bonds are weaker than reactants c.) Products have lower PE because product bonds are weaker than reactants d.) Products have higher PE because product bonds are stronger than reactantsarrow_forward
- Please complete these reactions: 1. но Kmno, он Heat 2. Kmno, Нeatarrow_forwardConsider the reaction conditions provided in the question(s) below question. Determine the type of reaction that will occur. Identify the role of aqueous sodium bicarbonate in this reaction. Br NaHCOg heat A) strong base B) weak base or nucleophile C) electrophile D) solventarrow_forwardOsO4 (cat) + OOH H₂SO4arrow_forward
- Synthesis of Benzoic Acid Is the reaction between sodium benzoate and hydrochloric acid exothermic or endothermic? What type of reaction is this? What are the products? Why is ice cold water rather than room temperature or warm water used to wash the remainder of the benzoic acid out of the beaker?arrow_forwardBriefly explain why alcoholic solutions (alcoholic NH2OHꞏHCl, alcoholic KOH, alcoholic HCl) are used in the hydroxamic acid test for esters, what happens if we use other solutions?arrow_forwardPredict the products (if any) of the following acid–base reactions.(a) acetic acid + ammoniaarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY