
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14.21, Problem 39P
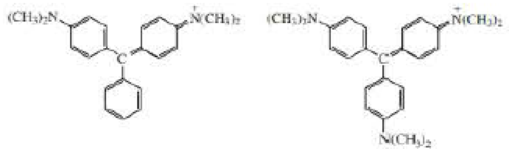
a. At pH = 7 one of the ions shown here is purple and the other is blue. Which is which? (Hint: refer to the color spectrum in figure 13.8 on page 584.)
b. What would be the difference in the colors or the compounds at pH = 3?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. The pH of blood is buffered by a mixture of HCO3- (bicarbonate) and H2CO3 (carbonic acid).i. Write out an equation that shows a reaction of H+ with HCO3-iii. Recall that H2CO3 (aq) decomposes readily. Write a chemical equation to describe this decomposition.
The pH of blood is buffered by a mixture of HCO3- (bicarbonate) and H2CO3 (carbonic acid).
Write out an equation that shows a reaction of H+ with HCO-3.
Recall that H2CO3 (aq) decomposes readily. Write a chemical equation to describe this decomposition.
Consider your response to the last question and explain how bicarbonate and carbonic acid are generated by the dissolving of CO2 in water (Hint: The reaction for “ii” is reversible.)
23. lodide ion (1) is the conjugate base of hydroiodic acid (HI). Why doesn't sodium iodide cause an
appreciable increase in the pH of water when it dissolves into Na* and I ions?
(a) The Na' is acidic and neutralizes the basicity of I
(b) It is a stronger acid than it is a base in water, so Nal actually lowers the pH when dissolved.
(c) There are no H₂O* ions present in the solution that could react with I.
(d) At least a small amount of HI must be present in order for I to act as a base to raise the pH.
(e) HI is a strong acid that reacts completely, which makes I a correspondingly weak base.
24. K, for methylamine (CH3NH₂) is the equilibrium constant for which reaction?
(a) CHÍNH, + HO
- CHÍNH + HO
CH₂NH3 + OH*
= CHÍNH + H2O
CH₂NH™ + H₂O
(b) CH₂NH₂ + H₂O
(C) CHÍNH, + HO
(d) CH₂NH₂ + OH
(e) None of the above
Consider the titration of 50.0 mL 0.1250 M KOH with 0.07500 HClO4 M.
a. Write the balanced reaction which occurs during the titration:Reaction:
b. Determine the pH of the solution before the titration begins.
c. Determine the pH of the solution after the addition of 40.0mL acid.
d. Determine the pH of the solution at the equivalence point.
e. Determine the pH of the solution after 125.0mL acid has been added.
Chapter 14 Solutions
Organic Chemistry
Ch. 14.1 - Which of the following fragments produced in a...Ch. 14.2 - What distinguishes the mass spectrum of...Ch. 14.2 - What is the most likely m/z value for the base...Ch. 14.3 - Prob. 5PCh. 14.3 - If a compound has a molecular ion with an...Ch. 14.3 - a. Suggest possible molecular formulas for a...Ch. 14.3 - Identify the hydrocarbon that has a molecular ion...Ch. 14.4 - Predict the relative intensities of the molecular...Ch. 14.5 - Which molecular formula has an exact molecular...Ch. 14.5 - Prob. 11P
Ch. 14.6 - Sketch the mass spectrum expected for...Ch. 14.6 - The mass spectra of 1-methoxybutane,...Ch. 14.6 - Prob. 14PCh. 14.6 - Identify the ketones responsible for the mass...Ch. 14.6 - Prob. 16PCh. 14.6 - Using curved arrows, show the principal fragments...Ch. 14.6 - The reaction of (Z)-2-pentene with water and a...Ch. 14.9 - Prob. 19PCh. 14.9 - Prob. 20PCh. 14.9 - Prob. 21PCh. 14.13 - Prob. 22PCh. 14.14 - Which occur at a larger wavenumber: a. the C O...Ch. 14.14 - Prob. 24PCh. 14.14 - Prob. 25PCh. 14.14 - Rank the following compounds from highest...Ch. 14.14 - Which shows an O H stretch at a larger...Ch. 14.15 - Prob. 28PCh. 14.15 - a. An oxygen-containing compound shows an...Ch. 14.15 - Prob. 30PCh. 14.15 - For each of the following pair of compounds, name...Ch. 14.16 - Which of the following compounds has a vibration...Ch. 14.16 - Prob. 33PCh. 14.17 - A compound with molecular formula C4H6O gives the...Ch. 14.19 - Prob. 35PCh. 14.19 - Prob. 36PCh. 14.20 - Predict the max of the following compound:Ch. 14.20 - Prob. 38PCh. 14.21 - a. At pH = 7 one of the ions shown here is purple...Ch. 14.21 - Prob. 40PCh. 14.22 - Prob. 41PCh. 14.22 - Prob. 42PCh. 14 - In the mass spectrum of the following compounds,...Ch. 14 - Prob. 44PCh. 14 - For each of the following pairs of compounds,...Ch. 14 - Draw structures for a saturated hydrocarbon that...Ch. 14 - a. How could you use IR spectroscopy to determine...Ch. 14 - Assuming that the force constant is approximately...Ch. 14 - In the following boxes, list the types of bonds...Ch. 14 - A mass spectrum shows significant peaks at m/z. =...Ch. 14 - Prob. 51PCh. 14 - Prob. 52PCh. 14 - Prob. 53PCh. 14 - How can you use UV spectroscopy to distinguish...Ch. 14 - Rank the following compounds from highest...Ch. 14 - Rank the following compounds from highest...Ch. 14 - What peaks in their mass spectra can be used to...Ch. 14 - Each of the IR spectra shown below is accompanied...Ch. 14 - Prob. 59PCh. 14 - Prob. 60PCh. 14 - How can IR spectroscopy distinguish between...Ch. 14 - 62. Draw the structure of a carboxylic acid that...Ch. 14 - Prob. 63PCh. 14 - Give approximate wavenumbers for the major...Ch. 14 - Prob. 65PCh. 14 - Prob. 66PCh. 14 - Prob. 67PCh. 14 - The IR spectrum of a compound with molecular...Ch. 14 - Which one of the following live compounds produced...Ch. 14 - Prob. 70PCh. 14 - Phenolphthalein is an acid-base indicator. In...Ch. 14 - Which one of the following five compounds produced...Ch. 14 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When a mixture containing cations of Analytical Groups I–III is treated with H 2S in acidic solution, which cations are expected to precipitate? a. Analytical Group I only b. Analytical Group II only c. Analytical Groups II and III d. Analytical Group III only e. Analytical Groups I and IIarrow_forward14. Which is the Bronsted base in the equation? [Fe(H20)61*3 + H20 = [Fe(H2O)5OH*2 + H30* [Fe(H2O)5OH*2 А. [Fe(H2O)6]*3 В. С. H20 H30* D.arrow_forward5.What are the limitations of modern periodic table you think? Write in “BULLET POINTS”. An alkaline solution was prepared with LiOH in such a way that 0.65 g dry LiOH was directly mixed with 0.6 M 650 mL LiOH solution and finally 350 mL more water was added to it. Find the final pH of the solution. You need to consider 100% dissociation of LiOH. [Ref: 7Li, 1H, 16O]arrow_forward
- We have seen an introductory definition of an acid: An acid is a compound that reacts with water and increases the amount of hydronium ion present. In the Chapter on acids and bases, we saw two more definitions of acids: a compound that donates a proton (a hydrogen ion, H+) to another compound is called a Bronsted-Lowry acid, and a Lewis acid is any species that can accept a pair of electrons. Explain why the introductory definition is a macroscopic definition, while the Bronsted-Lomy definition and the Lewis definition are microscopic definitions.arrow_forwardWhat are the equilibrium concentration of H3O+, CN and HCN in a 0.025 M solution of HCN? What is the pH of the solution?arrow_forward1. How many moles of electrons are transferred overall? 2. If E°cell = +1.76 V and Ecell = +1.25 V and [VO²⁺], [VO₂⁺] and [Zn²⁺] are all 1.0 M, what is the pH of the reaction? 4.31 0.69 1.70 3.61 None of the given.arrow_forward
- * OWLV2 | Online teaching X w.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take [Review Topics] [References] Use the References to access important values if needed for this question. Which of the following can behave as Bronsted-Lowry bases in aqueous solution? O so, O NaOH O H,S O KOH O None of the Above Retry Entire Group 8 more group attempts remaining Submit Answerarrow_forwardD. The element Radon has the symbol Cl452, what is its Atomic Number? How many Neutrons does it have? E. What type of radioactive decay is caused when there are too many protons in the nucleus? and What type of radioactive decay is caused when there are too many neutrons in the nucleus? F. What is the concentration of hydronium ions in a solution at 25°C with pH 4.282?arrow_forward6. Both CO3 and HCO3 respond to the usual carbonate ion test. Give specific procedures by which you could distinguish between these ions in an unknown. (Hint: Consider the pH of the solution.) 2-arrow_forward
- Can an acidic solution have a measurable alkalinity? Explain with a concrete example.arrow_forwardBased on the reaction, is it safe to use muriatic acid to clean limestone tiles? Explain.arrow_forward2. Ni(P(0-C6H4Me)3)2Cl2 is strongly paramagnetic, while Ni(PMe3)2Cl2 is diamagnetic (see the structures - note that they are not shown with any three dimensional notation). Suggest a possible explanation, and show how it is compatible with their different magnetic properties. -Ph -P Ph Ph Ph Ph Ph .Ni CI CI P Ni CI CIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Acid-base Theories and Conjugate Acid-base Pairs; Author: Mindset;https://www.youtube.com/watch?v=hQLWYmAFo3E;License: Standard YouTube License, CC-BY
COMPLEXOMETRIC TITRATION; Author: Pikai Pharmacy;https://www.youtube.com/watch?v=EQxvY6a42Dw;License: Standard Youtube License