
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14.20, Problem 37P
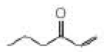
Predict the λmax of the following compound:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. Which of the following compounds would you expect to show ultraviolet absorptions in the 200
to 400 nm range?
(a)
(b)
(c)
CN
(d)
(e)
CH3
(f)
HO.
H.
Shown below is the ¹H-NMR spectrum of a compound with the formula C5H10O2.
2H
Peak Splitting
quartet
quartet
triplet
triplet
1
2
3
4
a b c d e a b
CH3CH₂CH₂CH₂COH
2
A
2H
PPM
Choose from the constitutional isomers below to assign a structure to this spectrum.
O
B
d
CH3CH₂CH₂COCH3
3
3H
4
3H
a b || c
CH3CH₂COCH₂CH3
C
(Choose the letter corresponding to the correct structure from the drop-down list provided.)
Correct Structure: C
Assign signal number 3 (indicated as a red number on the spectrum above) to its corresponding hydrogen(s) (shown as a red lower-case letter on the structure above).
(Write the letter of the hydrogen (or set of equivalent hydrogens) in the box provided, e.g., a)
Signal number 3 corresponds to hydrogen(s):
Previous
Next
9. In the following pairs of compounds, describe whether there should be an increase in the
wavelength of maximum absorption and whether there should be an increase in absorption
intensity in going from the first compound to the second:
(a)
00-89
Chapter 14 Solutions
Organic Chemistry
Ch. 14.1 - Which of the following fragments produced in a...Ch. 14.2 - What distinguishes the mass spectrum of...Ch. 14.2 - What is the most likely m/z value for the base...Ch. 14.3 - Prob. 5PCh. 14.3 - If a compound has a molecular ion with an...Ch. 14.3 - a. Suggest possible molecular formulas for a...Ch. 14.3 - Identify the hydrocarbon that has a molecular ion...Ch. 14.4 - Predict the relative intensities of the molecular...Ch. 14.5 - Which molecular formula has an exact molecular...Ch. 14.5 - Prob. 11P
Ch. 14.6 - Sketch the mass spectrum expected for...Ch. 14.6 - The mass spectra of 1-methoxybutane,...Ch. 14.6 - Prob. 14PCh. 14.6 - Identify the ketones responsible for the mass...Ch. 14.6 - Prob. 16PCh. 14.6 - Using curved arrows, show the principal fragments...Ch. 14.6 - The reaction of (Z)-2-pentene with water and a...Ch. 14.9 - Prob. 19PCh. 14.9 - Prob. 20PCh. 14.9 - Prob. 21PCh. 14.13 - Prob. 22PCh. 14.14 - Which occur at a larger wavenumber: a. the C O...Ch. 14.14 - Prob. 24PCh. 14.14 - Prob. 25PCh. 14.14 - Rank the following compounds from highest...Ch. 14.14 - Which shows an O H stretch at a larger...Ch. 14.15 - Prob. 28PCh. 14.15 - a. An oxygen-containing compound shows an...Ch. 14.15 - Prob. 30PCh. 14.15 - For each of the following pair of compounds, name...Ch. 14.16 - Which of the following compounds has a vibration...Ch. 14.16 - Prob. 33PCh. 14.17 - A compound with molecular formula C4H6O gives the...Ch. 14.19 - Prob. 35PCh. 14.19 - Prob. 36PCh. 14.20 - Predict the max of the following compound:Ch. 14.20 - Prob. 38PCh. 14.21 - a. At pH = 7 one of the ions shown here is purple...Ch. 14.21 - Prob. 40PCh. 14.22 - Prob. 41PCh. 14.22 - Prob. 42PCh. 14 - In the mass spectrum of the following compounds,...Ch. 14 - Prob. 44PCh. 14 - For each of the following pairs of compounds,...Ch. 14 - Draw structures for a saturated hydrocarbon that...Ch. 14 - a. How could you use IR spectroscopy to determine...Ch. 14 - Assuming that the force constant is approximately...Ch. 14 - In the following boxes, list the types of bonds...Ch. 14 - A mass spectrum shows significant peaks at m/z. =...Ch. 14 - Prob. 51PCh. 14 - Prob. 52PCh. 14 - Prob. 53PCh. 14 - How can you use UV spectroscopy to distinguish...Ch. 14 - Rank the following compounds from highest...Ch. 14 - Rank the following compounds from highest...Ch. 14 - What peaks in their mass spectra can be used to...Ch. 14 - Each of the IR spectra shown below is accompanied...Ch. 14 - Prob. 59PCh. 14 - Prob. 60PCh. 14 - How can IR spectroscopy distinguish between...Ch. 14 - 62. Draw the structure of a carboxylic acid that...Ch. 14 - Prob. 63PCh. 14 - Give approximate wavenumbers for the major...Ch. 14 - Prob. 65PCh. 14 - Prob. 66PCh. 14 - Prob. 67PCh. 14 - The IR spectrum of a compound with molecular...Ch. 14 - Which one of the following live compounds produced...Ch. 14 - Prob. 70PCh. 14 - Phenolphthalein is an acid-base indicator. In...Ch. 14 - Which one of the following five compounds produced...Ch. 14 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- List the HOMO and LUMO for each of the following molecules. Please choose from one of these options for each assignment: nonbonding, o,o*,π, or T*. It's not necessary in this question to specify the specific atoms involved. (a) H3C. H₂C-N H3C CH3 HOMO: LUMO: (b) HOMO: LUMO:arrow_forwardNicotine is a diamino compound isolated from dried tobacco leaves. Nicotine has two rings and M+=162.1157 by high-resolution mass spectrometry. Give a molecular formula for nicotine, and calculate the number of double bonds.arrow_forwardC5H10O2 H Determine H and C Spectra for the compundarrow_forward
- 12.34 How would you use infrared spectroscopy to distinguish between the follow- ing pairs of constitutional isomers? (a) CH3C=CCH3 文 and CH3CH2C=CH (b) CH3CH=CHCH3 and CH3CH,CH=CH2 (c) H2C=CHOCH3 CH3CH2CHO andarrow_forwardPredict the splitting patterns you would expect for each proton in the following molecules.(a)CHBr2CH3 (b)CH3OCH2CH2Br (c)ClCH2CH2CH2Cl Draw structures for compounds that meet the following descriptions:(a) C2H6O; one singlet(b) C3H7Cl; one doublet and one septet(c) C4H8Cl2O; two tripletsarrow_forward2. Predict the NMR spectra of the following compounds: a) b) ΟΗarrow_forward
- opic 1: What IR and H-NMR peaks would you expect to see in methyl 3-oxohexanoate(see p559, attached image)? Include band position, chemical shift, splitting in your response. مللarrow_forwardPropose possible molecular formulas for a compound with a molecular ion at m/z = 154.arrow_forwardG 8 HR201403000NS 18) The partial chemical structure and ¹H NMR spectrum of a compound with the formula C4H10O. Determine which of the following structures fit the spectrum (A-D). Provide a brief rationalization of the structure. 4H B) Peak 1: Peak 2: () HE 3.47 ppm 1.21 ppm 6H Triplet D) XOH I 0 Quartet ppm A) 4 Identity of Unknown: Explanation: WASTOR SERVICES FOR CHILDREN&FAMILIES wwww..arrow_forward
- This question has multiple parts. Work all the parts to get the most points. a Give the structure for the compound with the empirical formula C3H3O2 and the following spectral data: 1Η ΝMR Singlet at 3.7 ppm (3H); multiplet at 7.2 ppm (5H) IR 2850 cm1 (sharp), 1720 cm-1, 1600 cm-1, 1503 cm! 100- 105 80 77 60- M: 136 40- ae 20- 40 80 120 160 miz % Relative abundancearrow_forwardEstimate the relative contribution of the members in each set of contributing structures. (a) :: :O: H-C C-H H-C=C-H H. (b) O: H-C O: H-C=0 CIHarrow_forward5. Identify the number of π electrons in each of the following compounds: (a) (b) (d) (e) © give detailed Solution with explanation needed of all options, don't give Handwritten answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY