
Concept explainers
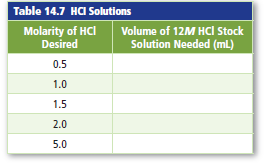
Stock solutions of HCl with various molarities are frequentlyprepared. Complete Table 14.7 by calculatingthe volume of concentrated, or 12M, hydrochloric acidthat should be used to make 1.0 L of HCl solution witheach molarity listed.
Interpretation:
Volume of the stocksolutions have to be calculated.
Concept introduction:
The equation for dilution is
M1V1=M2V2Stocksolution=Dilutedsolution
Concentrated solutions of known molarities are known as stock solution.
Where,
M1 = molarity of the stock solution V1 = volume of stock solution
M2 = molarity of the diluted solution V2 = volume of diluted solution
Answer to Problem 75A
| Molarity of HCl desired | Volume of 12M HCl stock solution needed (mL) |
| 0.5 | 41.7 |
| 1.0 | 83.3 |
| 1.5 | 125 |
| 2.0 | 167 |
| 5.0 | 417 |
Explanation of Solution
M1V1=M2V2Stocksolution=Dilutedsolution
Data given:
M1 = molarity of the stock solution = 12 M
M2 = molarity of the diluted solution
V2 = volume of diluted solution = 1 L
- Volume of 12M HCl stock solution needed to make 0.5 M diluted solution
- Volume of 12M HCl stock solution needed to make 1.0 M diluted solution
- Volume of 12M HCl stock solution needed to make 1.5 M diluted solution
- Volume of 12M HCl stock solution needed to make 2.0 M diluted solution
- Volume of 12M HCl stock solution needed to make 5.0 M diluted solution
V1 = M2V2M1V1 = 0.5 M × 1 L12 M = 0.0417 L= 41.7 mL
V1 = M2V2M1V1 = 1.0 M × 1 L12 M = 0.0833 L= 83.3 mL
V1 = M2V2M1V1 = 1.5 M × 1 L12 M = 0.125 L= 125 mL
V1 = M2V2M1V1 = 2.0 M × 1 L12 M = 0.167 L= 167 mL
V1 = M2V2M1V1 = 5.0 M × 1 L12 M = 0.417 L= 417 mL
Chapter 14 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Anatomy & Physiology (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology (11th Edition)
Microbiology with Diseases by Body System (5th Edition)
Applications and Investigations in Earth Science (9th Edition)
- Q4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br OH Brarrow_forwardQ7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability. a) H₂O, OH, CH3COOT b) NH3, H₂O, H₂Sarrow_forwardQ8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution reaction with CN as the nucleophile. Br A B NH2 LL F C D OH CI LLI E Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d). a) H "Cl D + -OCH 3 Page 3 of 5arrow_forward
- Q10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardComplete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- QUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forward
- Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forwardhelparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





