
Concept explainers
(a)
Interpretation:
Structure of the 3-methyl-3-pentanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as
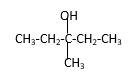
Answer to Problem 14.44P
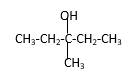
Explanation of Solution
Structure of the 3-methyl-3-pentanol contains five carbon length main carbon chain which connects to an alcohol group in the 3rd carbon of the main carbon chain. Furthermore, methyl group also connects to the 3rd position of the main carbon chain. And according to the structure it should be a tertiary alcohol.
According to the name, structure of the compound is as below;
(b)
Interpretation:
Structure of the 4-methyl-2-pentanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
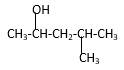
Answer to Problem 14.44P
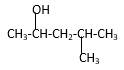
Explanation of Solution
Structure of the 4-methyl-2-pentanol consist one main C chain which contains five C atoms, and alcohol group is connected to the 2nd position of the main C ring. Methyl group is connects to the 4th position of the main C chain. And as per the name it should be a secondary alcohol.
Structure of the compound is as below;
(c)
Interpretation:
Structure of the 2,4-dimethyl-2-hexanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 14.44P
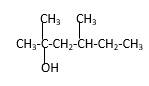
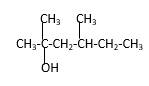
Explanation of Solution
Structure of the 2, 4-dimethyl-2-hexanol consist one main C chain which contains six C atoms, and alcohol group is connected to the 2nd position of the main C ring. Two methyl groups are connected to the 4th position and 2nd position of the main C chain. And as per the name it should be a tertiary alcohol.
Structure of the compound is as below;
(d)
Interpretation:
Structure of the 1,3-propanediol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 14.44P
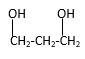
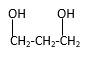
Explanation of Solution
Structure of the 4-methyl-2-pentanol consist one main C chain which contains five C atoms, and alcohol group is connected to the 2nd position of the main C ring. Methyl group is connects to the 4th position of the main C chain. And as per the name it should be a secondary alcohol.
Structure of the compound is as below;
(e)
Interpretation:
Structure of the 3,5-dimethylcyclohexanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 14.44P
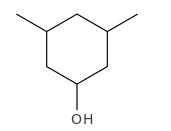
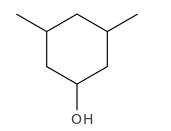
Explanation of Solution
Structure of the 3,5-dimethylcyclohexanol consist one main C ring which contains six C atoms, and alcohol group is connected to the 1st position of the main C ring. Two methyl groups are connected to the 3rd and 5th position of the main C ring. And as per the name it should be a secondary alcohol.
Structure of the compound is as below;
(f)
Interpretation:
Structure of the 4-methyl-2-pentanol should be drawn.
Concept Introduction:
Alcohols are the organic molecules which have OH group bonded to a tetrahedral carbon atom.
Longest carbon chain containing the carbon bonded to the OH group is named as an alkane and -e of the alkane replaced by the suffix -ol.
Numbering of main carbon chain is done in such a way so that OH group gets the lowest number.
When OH group is bonded to a ring, the ring is numbered beginning with the OH group and the 1 is normally omitted from the name. The ring is numbered in clockwise or anticlockwise by giving the lowest number to the next substitute.
Compounds which contains two OH groups are named as diols and when in nomenclature, -diol suffix is added to the end of the parent alcohol and position of the OH groups are used as prefix to indicate the location of the two OH groups.
Answer to Problem 14.44P
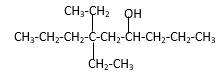
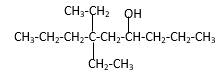
Explanation of Solution
Structure of the 4-methyl-2-pentanol consist one main C chain which contains five C atoms, and alcohol group is connected to the 2nd position of the main C ring. Methyl group is connects to the 4th position of the main C chain. And as per the name it should be a secondary alcohol.
Structure of the compound is as below;
Want to see more full solutions like this?
Chapter 14 Solutions
Connect 1-Semester Access Card for General, Organic, & Biological Chemistry
- Determine the IUPAC name of the following compound: O 3-methyl-2-butanol O 3-methyl-2-butene 2-methyl-2-butanol 2-methyl-3-butanol OH CH3-CH-CH-CH3 CH3arrow_forwardWhich of the following alcohols is least likely to be soluble in water? 3-pentanol 2-butanol 1,2-ethanediol 1,2,3-propanetriol Methanolarrow_forwardGive the IUPAC name for the organic compound formed when 1-propanol is dehydrated in the presence of an acid at 140 degrees Celsius.arrow_forward
- What is the IUPAC name of this compound? OH CH3 - C - CH3 CH3 O2-propanol O 2-methyl-2-propanol O butanol O 2-methylbutanol O propanolarrow_forwardGive the common name for NN OH T CH3-CH₂-C-N-CH₂-CH3 Spell out the common name of the compound. Give the IUPAC name for 0 CH₂ || CH₂-C-N-CH₂-CH₂-CH₂, Spell out the IUPAC name of the compound. diethylamine Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. HE CONT HOarrow_forwardSelect the correct IUPAC name for the compound. H The IUPAC name is: O 2-ethyl-2-phenylpropanal 2-methyl-2-phenylbutanal O 3-methyl-3-phenylbutan-4-al O 2-phenyl-2-methylbutanalarrow_forward
- Which is the correct IUPAC name for the following structure? T OH 1,1-diethyl-1-propanol 2-ethyl-3-pentanol 3-ethyl-3-pentanol t-heptanolarrow_forwardComplete the following: IUPAC Name Chemical structure Common use Ethyl alcohol Isopropyl alcohol Ethylene glycol Benzyl Alcohol Phenol Resorcinol Salicyclic acid Thank you in advance!arrow_forwardWhat is the correct IUPAC name of the structure below?arrow_forward
- Draw the structural formula for each alcohol before you answer the questions. 2- methyl -1- propanol 2-butanol 2-methyl-2-propanolarrow_forwardWrite a condensed structural formula for each of the following alcohols. a. 2-Methyl-2-heptanol b. 3-Ethyl-2-pentanol c. 3-Phenyl-1-butanol d. 3,5-Dimethylcyclohexanolarrow_forwardArrange these compounds in order of increasing boiling point. (a) 1-butanol, butane, diethylether (b) hexane, 1-hexanol, dipropyletherarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





