
Concept explainers
(a)
Interpretation:
The bond length of the indicated
Concept introduction:
Weak resonance contributors give some single-bond character to the two terminal
Answer to Problem 14.33P
In
Explanation of Solution
The bond length of
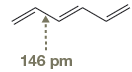
The normal
The amount of shortening of
(b)
Interpretation:
The bond length of the indicated
Concept introduction:
Resonance contributors give some double-bond character to the
Answer to Problem 14.33P
The occupied
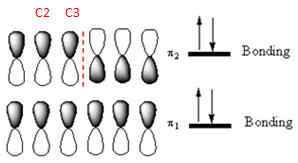
Explanation of Solution
The bond length of
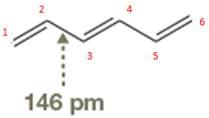
The occupied
Out of these, the occupied

The occupied
(c)
Interpretation:
The bond length of the indicated
Concept introduction:
The highest energy MO of
Answer to Problem 14.33P
If an electron were promoted from the HOMO to the LUMO, the length of the
Explanation of Solution
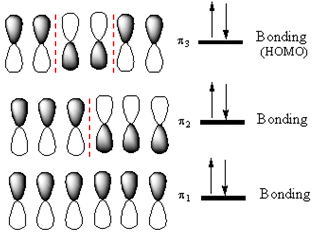
The
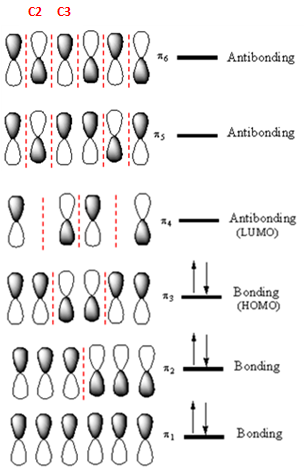
If an electron is promoted from HOMO (
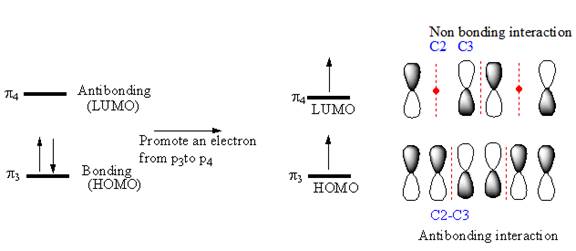
Therefore, the removal of an electron from the HOMO contributes to a shorter C2-C3 bond.
If an electron were promoted from the HOMO to the LUMO, the length of the
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry: Principles And Mechanisms
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning



