
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.28P
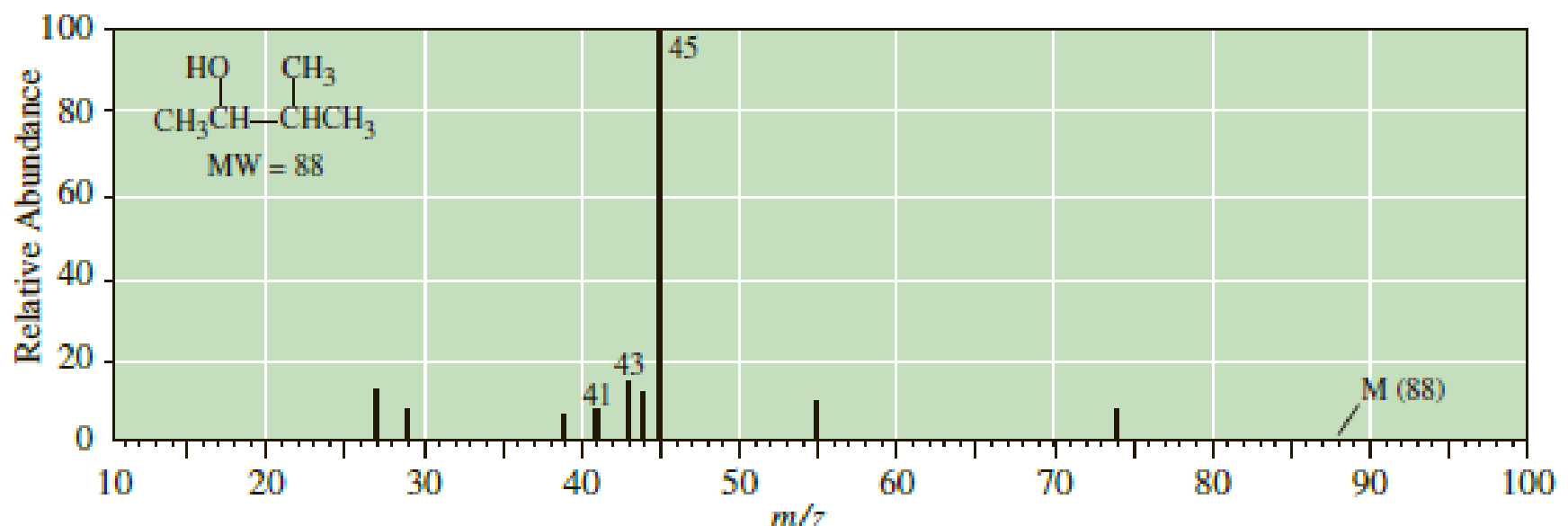
Following is the mass spectrum of 3-methyl-2-butanol. The molecular ion m/z 88 does not appear in this spectrum. Propose structural formulas for the cations of m/z 45, 43, and 41.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A low-resolution mass spectrum of the neurotransmitter dopamine gave a molecular ion at m/z = 153. Two possible molecular formulas for this molecular ion are C8H11NO2 and C7H11N3O. A high-resolution mass spectrum provided an exact mass at 153.0680. Which of the possible molecular formulas is the correct one?
The mass spectrum of n-octane shows a prominent molecular ion peak (m>z 114). There is also a large peak at m>z 57,but it is not the base peak. The mass spectrum of 3,4-dimethylhexane shows a smaller molecular ion, and the peak atmass 57 is the base peak. Explain these trends in abundance of the molecular ions and the ions at mass 57, and predict theintensities of the peaks at masses 57 and 114 in the spectrum of 2,2,3,3-tetramethylbutane.
The mass spectrum of an aldehyde shows a parent peak at m/z = 58 and a base peak at m/z = 29. Propose a structure, and identify the
two species whose m/z values were listed.
Name the compound in the box below.
Chapter 14 Solutions
Organic Chemistry
Ch. 14.2 - Calculate the nominal mass of each ion. Unless...Ch. 14.3 - Propose a structural formula for the cation at m/z...Ch. 14.3 - The low-resolution mass spectrum of 2-pentanol...Ch. 14 - Draw acceptable Lewis structures for the molecular...Ch. 14 - The molecular ion for compounds containing only C,...Ch. 14 - For which compounds containing a heteroatom (an...Ch. 14 - The so-called nitrogen rule states that if a...Ch. 14 - Prob. 14.8PCh. 14 - Prob. 14.9PCh. 14 - Prob. 14.10P
Ch. 14 - Determine the probability of the following in a...Ch. 14 - The molecular ions of both C5H10S and C6H14O...Ch. 14 - Prob. 14.13PCh. 14 - Carboxylic acids often give a strong fragment ion...Ch. 14 - For primary amines with no branching on the carbon...Ch. 14 - Prob. 14.16PCh. 14 - A characteristic peak in the mass spectrum of most...Ch. 14 - Predict the relative intensities of the M and M +...Ch. 14 - The mass spectrum of compound A shows the...Ch. 14 - The mass spectrum of compound B, a colorless...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - The molecular ion in the mass spectrum of...Ch. 14 - Prob. 14.24PCh. 14 - Following is the mass spectrum of 1-bromobutane....Ch. 14 - Following is the mass spectrum of...Ch. 14 - Following is the mass spectrum of an unknown...Ch. 14 - Following is the mass spectrum of...Ch. 14 - Prob. 14.29PCh. 14 - Following are mass spectra for the constitutional...Ch. 14 - 2-Methylpentanal and 4-methyl-2-pentanone are...Ch. 14 - Prob. 14.32PCh. 14 - Account for the presence of the following peaks in...Ch. 14 - All methyl esters of long-chain aliphatic acids...Ch. 14 - Propylbenzene, C6H5CH2CH2CH3, and isopropyl...Ch. 14 - Account for the formation of the base peaks in...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
16.43 The following pictures represent solutions at various stages in thetitration of a weak diprotic acid with...
Chemistry (7th Edition)
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
Give the IUPAC name for each compound.
Organic Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is the mass spectrum of bromocyclopentane. The molecular ion m/z 148 is of such low intensity that it does not appear in this spectrum. Assign structural formulas for the cations of m/z 69 and 41.arrow_forwardFollowing is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122. Suggest a structure for this compound. (Data from http://webbook.nist.gov/chemistry/.)arrow_forwardWrite molecular formulas for compounds that show the following molecular ions in their high-resolution mass spectra, assuming that C, H, N, and O might be present. The exact atomic masses are: 1.007 83 (1H), 12.000 00 (12C), 14.003 07 (14N), 15.994 91 (16O). (a) M+=98.0844 (b) M+=123.0320arrow_forward
- A compound containing only carbon, nitrogen, oxygen, and hydrogen contains four carbon atoms. If the M+ peak in its mass spectrum appears at m/z = 87, then how many nitrogen atoms does it contain?arrow_forwardAssigning Possible Structures to Fragments in a Mass Spectrum The mass spectrum of 2,3-dimethylpentane [(CH3)2CHCH(CH3)CH2CH3] shows fragments at m/z = 85 and 71. Propose possible structures for the ions that give rise to these peaks.arrow_forwardc) The mass spectrum of 2,3-dibromopentane (CH; CHBr CHBr CH₂ CH3) includes the following peaks. Mass number (m/z) Relative abundance 15 29 107 109 199 201 203 i. What is the mass number of molecular ion, [CH; CHBr CHBr CH₂ CH₂]? Show your working. ii. Identify the molecular formula (including isotopic composition where relevant) of the 6 peaks. a b d 22 e f Mass number (m/z) 15 29 107 109 199 100 39 44 45 0.3 0.6 0.3 201 203 Molecule formula [CH₂]arrow_forward
- The mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. One appears at m/z = 111 and the other appears at m/z = 97. Determine the identity and structure of each of these fragmentsarrow_forwardThe mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. Kne appears af m/z=111 and the other appears at m/z=97. Identify the structure of each of these fragments.arrow_forwardFollowing is the mass spectrum of 3-methyl-2-butanol. The molecular ion mlz 88 does not appear in this spectrum. Propose structural formulas for the cations of mlz 45, 43, and 41. 100 45 CH3 CH,CH-CHCH, MW - 88 но 80 60 20 M (88) 10 20 30 40 50 60 70 80 90 100 Relative Abundancearrow_forward
- A mass peak at m/z = 59 appears in the mass spectrum of an amide, C5H11NO. Draw the structure of a molecule that is consistent with this result.arrow_forwardLabel the molecular ion, the base peak, and the M + 1 peak in the mass spectrum of pentane (C5H12).arrow_forwardFollowing is the mass spectrum of an unknown compound. The two highest peaks are at mlz 120 and 122. Suggest a structure for this compound. (Data from http://webbook .nist.gov/chemistry/.) 100 41 80 20 120 122 0 rt 10 20 30 40 60 70 110 140 80 90 m/z 50 100 120 130 150 160 Relative Abundancearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY