
Interpretation:
In which rule expect for the
Concept Introduction:
Nitrogen rule: The nitrogen rule states, that a molecule that has no or even number of nitrogen atoms has an even nominal mass, whereas a molecule that has a odd number of nitrogen atoms has an odd nominal mass.
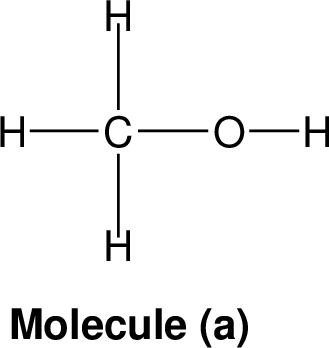 | |
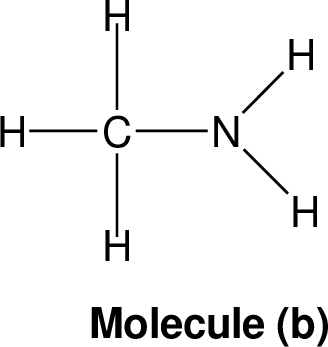 |
Nominal mass: The nominal mass for an element is the mass number of it is most abundant naturally occurring stable isotope and for an ion or given molecule the nominal mass is the sum of the nominal masses of the constituent atoms.
Example: The exact mass of the most abundant
For example hydrogen
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
- The mass spectrum of benzocaine is given below. Draw the structures of the fragment ions that give arise to the peaks at m/z= 165, 137, 120, and 92. 70eV Electron kanization Mass Spectrum Aonarce H2N benzocainearrow_forwardWhat is not true about mass spectrometry? Only the molecular ion and cationic fragments are deflected, and they are then separated by their mass-to-charge ratio (m/z) In a mass spectrometer, a compound is first vaporized and then bombarded with electromagnetic radiation which generates a radical cation that is symbolized by (M) + A molecular ion is often very unstable and susceptible to fragmentation In a mass spectrometer, a compound is converted into ions, which are then separated by a magnetic field. It is used to determine the molecular weight and molecular formula of a compound.arrow_forward4. Why does a carbon to oxygen double bond absorption band have a greater intensity than a carbon to carbon double bond absorption band?arrow_forward
- Can IR spectroscopy be used for quantitative analysis? >No, because IR spectroscopy only records vibrational frequency of bonds. >No, because IR spectroscopy only identifies the types of covalent bonds. >Yes, because the transmittance of the analyte is proportional to concentration. >Yes, because the absorbance of the analyte is proportional to concentration.arrow_forwardWhat is the molecular mass observed for Man9(GlcNAc)2 if it has a mass spectrometry ionization that imparts a +3 charge state? .arrow_forwardGiven that Kb for (CH3)3N is 6.3 × 10-5 at 25 °C, what is the value of Ka for (CH3)3NH at 25 °C?arrow_forward
- Analyze the mass spect (including fragmentations) for the molecular structure of vanillin (C8H8O3). M=152arrow_forwardThe IR frequency of C≡N¯ in fac-[IrCl3(C≡N)3] is 2200 cm-1. Estimate the IR frequency of C≡N¯ for fac-[IrF3(C≡N)3] with an explanation.arrow_forward13C NMR spectroscopy provides valuable information about the environments of a molecule's carbon atoms. Since carbon atoms are often connected to hydrogen atoms, which could split the carbon signal through spin-spin coupling, the coupling between C and H is often "turned off" through the use of broadband decoupling, causing each C signal to appear as a singlet. Draw an isomer of C5H11Cl that would be expected to have four resonances in its 13C NMR spectra.arrow_forward
- How can you distinguish aldehydes, ketones, and carboxylic acids from each other using IR spectra? Explain using specific examples.arrow_forwardMolecular Recognition (Supramolecular Chemistry) in organic chemistry deals with the "lock and key " mechanisms that form new molecules. However, little is known of this subject when moleules go beyond 1500 daltons (g/mole) for example macromolecules of polyolefins (polyethylene, polypropylene), thermoplastic polyesters and polyamides (nylon). It is understood that pre-directional H bonding is the mechanism of molecular recognition of macromolecules. Question: What is the immediate and post manifestation of inducing molecular recognition to condensation polymers and ring opening polymerization polymers like thermoplastic polyesters (PET ), and Nylon 66, 6 etc. (polyamides)?arrow_forwardWrite the resonance forms, if resonance effect is present, of the molecules below and report, the form (s) that present the most important statistical weight: N2 CO2H `NH2arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
