
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.13QAP
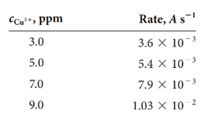
Copper(II) forms a 1:1 complex with the organic complexing agent R in acidic medium. The formation ofthe complex can be monitored by spectrophotometry at 480 nm. Use the following data collected under pseudo-first-order conditions to construct a calibration curve of rate versus concentration of R. Find the concentration of copper(II) in an unknown whose rate under the same conditions was 6.2 × 10- 3A s-1.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
. A student collects the following data for the absorbance of a complex measured in 1.00 cmspectrophotometric cells. The molar absorptivity (ε) of the complex under investigation is 2.25×103L/mol·cm. Calculate the concentration of the complex in solution at each time of the reaction.Time (min)MeasuredAbsorbanceComplex Concentration(M)0.000 1.50010.000 1.31320.000 1.12530.000 0.93840.000 0.750
11. A researcher wants to synthesize some pentaaminefluoromanganese(l) starting with
pentaaminechloromanganese(l). These are octahedral compounds. In order to identify the
mechanism of reaction the researcher increased the concentration of chloride and found
that no Mn(NH3)sF is formed.
Do you think this is a dissociative or associative mechanism?
Draw out the reaction steps.
What is the coordination number for the intermediate?
Use the equation: ln(k1/k2)=−Ea/R(1/T1−1/T2) to calculate the activation energy
k1 is 0.00316
k2 is 0.0106
R is 8.314
T2 is 298
T1 is 273
Chapter 14 Solutions
Principles of Instrumental Analysis
Ch. 14 - Prob. 14.1QAPCh. 14 - A 0.4740-g pesticide sample was decomposed by wet...Ch. 14 - Sketch a photometric titration curve for the...Ch. 14 - Prob. 14.4QAPCh. 14 - Prob. 14.5QAPCh. 14 - The accompanying data (1.00-cm cells) were...Ch. 14 - A 3.03-g petroleum specimen was decomposed by wet...Ch. 14 - Prob. 14.8QAPCh. 14 - Prob. 14.9QAPCh. 14 - The acid-base indicator HIn undergoes the...
Ch. 14 - Prob. 14.11QAPCh. 14 - Prob. 14.12QAPCh. 14 - Copper(II) forms a 1:1 complex with the organic...Ch. 14 - Aluminum forms a 1:1 complex with...Ch. 14 - Prob. 14.15QAPCh. 14 - Prob. 14.16QAPCh. 14 - Prob. 14.17QAPCh. 14 - Prob. 14.18QAPCh. 14 - Prob. 14.19QAPCh. 14 - Given the Information that...Ch. 14 - Prob. 14.21QAPCh. 14 - Mixing the chelating reagent B with Ni(II) forms...Ch. 14 - Prob. 14.23QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. A student collects the following data for the absorbance of a complex measured in 1.00 cm spectrophotometric cells. The molar absorptivity (ɛ) of the complex under investigation is 0.750 L/mol·cm. Calculate the concentration of the solution at each time of the reaction. Measured Complex Concentration Time (min) Absorbance (M) 0.000 1.500 10.000 1.313 20.000 1.125 30.000 0.938 40.000 0.750arrow_forward1. Reaction S: Cr(CO)s+ PH3 Cr(CO)s(PH3) + CO Reaction T: Mo(CO)s + Cl- Mo(CO):CI + CO (i) Choose the reaction that likely to proceed via associative mechanism. (ii) Explain your answer by elaborating the factors that favor the mechanism.arrow_forwardThe dissociation constant, Kd, for a particular repressor-operator complex is approximately 10-11 M. An E. coli cell with a volume of 2.0 x 10-12 mL contains 10 copies of the repressor. Calculate the cellular concentration of the repressor protein inside the cell. repressor protein concentration = Number M Given the concentration of repressor protein and its Kd value, select the statement below that is most likely correct. The operator site is almost always bound by the repressor. The concentration of repressor-operator complex is not affected by the concentration of repressor protein. The operator site is rarely bound by the repressor. The repressor is not able to bind to the operator site.arrow_forward
- Explain the mechanism of precipitation of the copper-ammonia complex in the presence of methanol?arrow_forwardGiving appropriate examples, explain how the two types of processes of adsorption (physisorption and chemisorption) are influenced by the prevailing temperature, the surface area of the adsorbent and the activation energy of the process?arrow_forwardThe aquation of tris-(1,10-phenanthroline) iron(II) in acid solution takes place according to the equation: Fe(phen),2+ + 3 H3O* + 3 H20→ Fe(H,O),2* + 3 phenH. If the activation energy, Ea, is 126 kJ/mol and the rate constant at 30°C is 9.8 x 103 min 1, what is the rate constant at 35°C? O 4.5 x 10 min 1 O 4.4 x 10 min 1 O 2.3 x 10 min 1 O 2.2 x 10 min 1arrow_forward
- 2. From the perspective of thermodynamics, the total free energy change of antibody (Ab) and antigen (Ag) interaction can be expressed as AG = -RTln(K·1M), where R = 8.3145 J/(mol·K) is the gas constant, T is the temperature in the unit of Kelvin (K = °C + 273.15), and K is affinity constant of Ab-Ag interaction. Note: 1M here means 1 Molar, so that affinity constant K has the unit of 1/M. A negative value of free energy change AG indicates energy release, while a positive value of free energy change AG indicates energy absorption. a) At body temperature T = 37°C, if we know the affinity constant K = 108/M, please compute the total free energy change AG. b) The presence of a single charged group on epitope or antigenic determinant of an antigen, typically increases the energy release by 20 kJ/mol for Ab-Ag interaction. Please compute thearrow_forwardw.com/ilm/takeAssignment/takeCovalentActivity.do?locator%3Dassignment-take References) Use the References to access important values if needed for this question. The decomposition of ammonia on a platinum surface at 856 °C NH3 1/2 N2 + 3/2 H2 is zero order in NH3 with a rate constant of 1.50x10 6 M s 1 If the initial concentration of NH3 is 1.28×10 2 M, the concentration of NH3 will be M after 7.54×103 seconds have passe Submit Answer Try Another Version 3 item attempts remainingarrow_forwardOligomeric proteins are common and commonly occur when binding a ligand. Consider a reaction where 2 molecules of A combine with B to form an A2B complex. No detectable AB complexes exist. Using your knowledge of equilibrium binding, show that the fraction of B bound (ie fraction of A2B from the total B) fits the following expression: fraction B bound = [?] 2 ?? + [?] 2arrow_forward
- For a reaction where Rate = k[CV]1[OH]0, what would be the effect of halving the hydroxide ion concentration while doubling the crystal violet concentration?arrow_forwardWhat is the activation energy (Qv) in eV/atom for a vancay formation if 10 moles of a metal has 2.3x10 ^ 13 vacancies at 425 C?arrow_forwardCalculate the activation energy for vacancy formatiın in Aluminuim, given that theequibilirium number of vacancies 500 C is 7.57x1023 m-3. The atomic weigth and density (500C) for aluminium are, respectively, 26.98 g/mol and 2.62 g/cm3.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License