
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 13.9, Problem 20P
Interpretation Introduction
Interpretation:
Compounds that are expected to undergo decarboxylation have to be identified.
Concept Introduction:
Carbon dioxide can be removed from carboxylic acid by heating, if the carbonyl group is present at the 3rd position.
Mechanism of decarboxylation is given below,
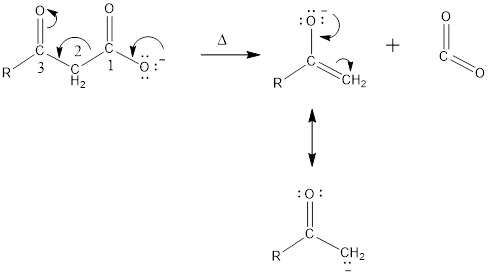
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following compounds will decarboxylate (lose carbon dioxide) in the presence ofaqueous acid and heat? Provide the product for one of the compounds that can be decarboxylated.
Which of the following materials will most likely form an ester too if subjected to the same process as aspirin synthesis?
A Benzoic Acid
B Benzamide
C Benzaldehyde
D Benzenol
Please give the reactions of the following of its esterification reaction:
1. benzoic acid
2. acetic acid + benzyl alcohol
Chapter 13 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 13.1 - Identify the most acidic hydrogen in each...Ch. 13.1 - Prob. 2PCh. 13.1 - Prob. 3PCh. 13.1 - Prob. 4PCh. 13.1 - Explain why HO cannot remove a proton from the...Ch. 13.2 - Prob. 6PCh. 13.2 - Prob. 7PCh. 13.3 - Prob. 8PCh. 13.3 - Prob. 9PCh. 13.3 - Prob. 10P
Ch. 13.4 - Prob. 11PCh. 13.5 - Prob. 12PCh. 13.5 - Prob. 13PCh. 13.6 - Prob. 14PCh. 13.7 - Prob. 16PCh. 13.8 - Prob. 17PCh. 13.8 - Prob. 18PCh. 13.8 - Prob. 19PCh. 13.9 - Prob. 20PCh. 13.10 - Propose a mechanism for the formation of...Ch. 13.10 - Prob. 22PCh. 13.10 - a. If the biosynthesis of palmitic acid were...Ch. 13 - Draw the enol tautomers for each of the following...Ch. 13 - Number the following compounds in order from...Ch. 13 - Prob. 26PCh. 13 - Explain why the pKa of a hydrogen bonded to the...Ch. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - Prob. 31PCh. 13 - Prob. 32PCh. 13 - Prob. 33PCh. 13 - Using cyclopentanone as the reactant, show the...Ch. 13 - Prob. 35PCh. 13 - Prob. 36PCh. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - Describe how the following compounds can be...Ch. 13 - Prob. 46PCh. 13 - Which would require a higher temperature:...Ch. 13 - Prob. 48PCh. 13 - Propose a mechanism for the following reaction:Ch. 13 - Show how the following compounds could be...Ch. 13 - Prob. 51PCh. 13 - Prob. 52P
Knowledge Booster
Similar questions
- Predict the products formed when cyclohexanone reacts with the following reagents.ethylene glycol and p-toluenesulfonic acidarrow_forwardWhat products are formed from the acid-catalyzed hydrolysis of the following esters?arrow_forwardWhat are all the possible electrophilic aromatic substitution reactions for the p-toluenesulfonic acid?arrow_forward
- draw the organic products you would expect to isolate, from the following reactions (after hydrolysis)arrow_forwardillustrate the chemical equation of N,N- Dimethyl aniline reacts with Diazotized Sulfanilic acid to form Methyl Orangearrow_forwardOut of acid chlorides (least stable), anhydrides, esters, and amides (most stable). True or false Acid chlorides would be the most reactive to hydrolysis and Amides would be the least reactivearrow_forward
- Which of the following reactions represents the synthesis of dibenzalacetone? Ph = phenylarrow_forwardWhich of the following compounds will NOT undergo nucleophilic substitution reaction? isopropanol Benzoyl chloride propylene oxide butanonearrow_forwardWhen a molecule has two esters, it can react via an intramolecular Claisen condensation. This is called a Dieckmann condensation. what would be the products of the following reaction (hint: what is going to happen to the protecting group in dilute aqueous acid?).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning