
Concept explainers
Draw the enol tautomers for each of the following compounds. If the compound has more than one enol tautomer, indicate which one is more stable.
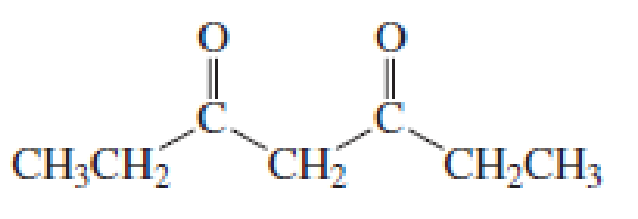
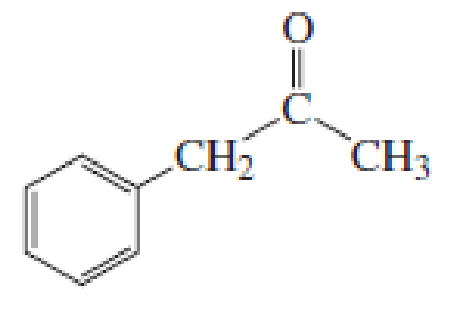
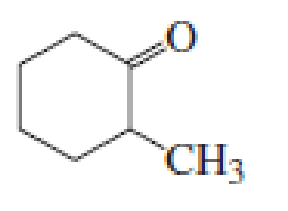
(a)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
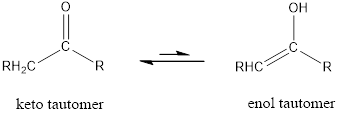
Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
Explanation of Solution
Given keto tautomer is,
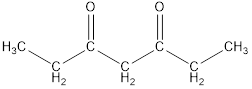
The only difference in keto-enol tautomer is the location of hydrogen and double bond.
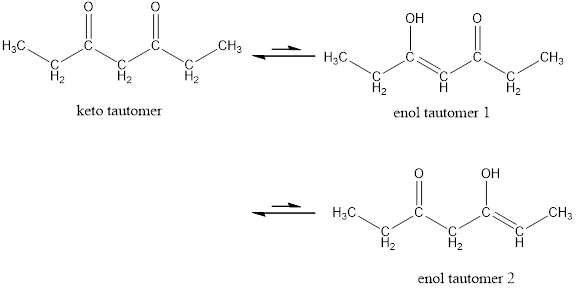
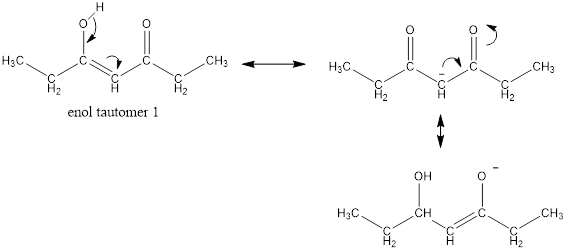
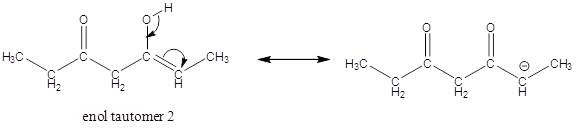
Enol tautomer 1 is more stable than enol tautomer 2.
Enol tautomer 1 can undergo delocalization and are more stable.
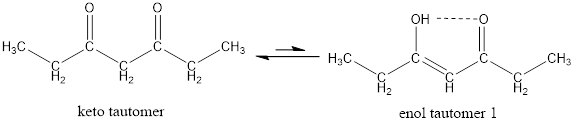
Thus enol tautomer 1 is more stable since it has more resonance structures and also possess intramolecular hydrogen bonding.
(b)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Resonance is an electron displacement effect for stabilizing a molecule through delocalization of bonding electrons in the pi orbital.
Delocalized electrons stabilize a compound. The extra stability gains from having delocalized electrons are called resonance stabilization or resonance energy.
Explanation of Solution
Given keto tautomer is,
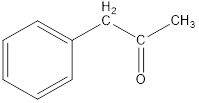
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
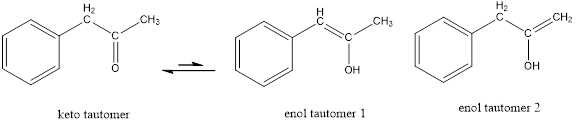
These tautomers undergo resonance and are shown below,
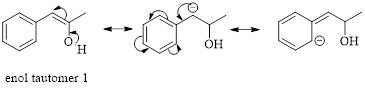
Enol tautomer 1 can undergo delocalization. Enol tautomer 1 is more stable than enol tautomer 2.
The enol tautomer 1 is more stable because there is a conjugation between the double bond and benzene ring. No such conjugation is possible in the enol tautomer 2.
(c)
Interpretation:
The enol tautomer of the given compound has to be drawn and more stable structure has to be identified.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form.
Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Enol tautomer is much less stable than the keto tautomer.
Enol tautomer is more stable when enol tautomer is aromatic or when the double bonds are conjugated.
Explanation of Solution
Given keto tautomer is,
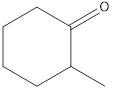
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
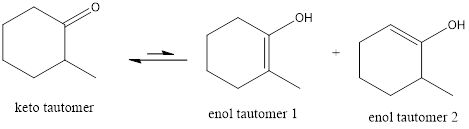

The alkene double bond formed by tautomer 1 is tetra-substituted which is stable than tautomer 2.
Hence, enol tautomer 1 is more stable.
Want to see more full solutions like this?
Chapter 13 Solutions
Essential Organic Chemistry (3rd Edition)
- Identify the best reagents to complete the following reaction. HO, CIarrow_forwardWhich of the following is a keto-enol tautomeric pair? o-Harrow_forwarda) Draw all resonance forms of 3-hydroxybenzaldehyde and of the corresponding conjugate base. W hich conclusions can you draw for its acidity (pKa) in comparison to phenol?| b) Draw all resonance forms of 3-hydroxybenzaldehyde and of the corresponding conjugate base. Which conclusions can you draw for its acidity (pKa) in comparison to phenol and 3-hydroxybenzaldehyde? c) Discuss the relative acidities of 2-hydroxybenzaldehyd und 4-hydroxybenzaldehyd.arrow_forward
- 6arrow_forwardAnswer the following question about curcumin, a yellow pigmentisolated from turmeric, a tropical perennial in the ginger family and aprincipal ingredient in curry powder. Most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols.arrow_forwardDraw the structure for the product formed in each of step of the following synthetic sequencesarrow_forward
- Draw the major product X when the given aldehyde is treated with ethylene glycol in acid.arrow_forward7) Provide a synthesis of the following compounds using the given starting material and any other reagents. so,H starting material final product он он starting material final productarrow_forwardDraw 2 enol forms of the following diketone.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
