
Basic Chemistry
6th Edition
ISBN: 9780134878119
Author: Timberlake, Karen C. , William
Publisher: Pearson,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 58UTC
Interpretation Introduction
Interpretation:
‘Reaction is exothermic or endothermic’ should be identified.
Concept introduction:
Equilibrium is the condition at which the rate of formation of product is equal to rate of disappearance of reactant.
Exothermic reaction, are the reaction which liberates energy and the temperature of the product is lower than reactants to proceed the reaction.
Endothermic reaction, are the reaction which absorbs energy and the temperature of the product is higher than reactants to proceed the reaction.
Given:
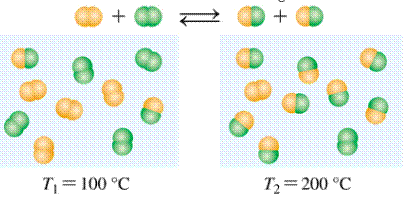
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
53. The combustion of glucose, CH1,O, forms carbon
dioxide gas and water vapour. When a 1.00 g sample
of glucose was burned, it raised the temperature
100.0 mL of water by 37.0 °C. (5.2, 5.3, 5.5) AA
(a) Write a balanced chemical equation for this reaction.
(b) Use AH to calculate the enthalpy change of this
of
reaction.
(c) Use bond energies in Table 1 on page 307 to
calculate the enthalpy change of this reaction.
The structural formula of glucose is shown in
Figure 1.
(7.6)Aluminum oxide decomposes to aluminum and oxygen gas by the following reaction.
Al2O3(s)- → 2Al(s) + 3/2 O2(g)
A Hrxn 1676 kJ
=
Is this reaction endothermic or exothermic?
[Choose ]
[Choose
1676 kJ
If 1.049 moles of aluminum was formed by the reaction,
how much heat (kJ) would be involved?
440 kJ
endothermic
1760 kJ
879 kJ
exothermic
(7.6)Classify each of the following change or reaction as endothermic or exothermic.
combustion of propane gas
[Choose]
[Choose
endothermic
vaporization of rubbing alcohol
exothermic
condensation of gas to liquid
[Choose]
Chapter 13 Solutions
Basic Chemistry
Ch. 13.1 - Prob. 1PPCh. 13.1 - Prob. 2PPCh. 13.1 - In the following reaction, what happens to the...Ch. 13.1 - Prob. 4PPCh. 13.1 - Prob. 5PPCh. 13.1 - Prob. 6PPCh. 13.2 - What is meant by the term reversible reaction?Ch. 13.2 - Prob. 8PPCh. 13.2 - Which of the following are at equilibrium? a. The...Ch. 13.2 - Which of the following are not at equilibrium? a....
Ch. 13.2 - 13.11 The following diagrams show the chemical...Ch. 13.2 - Prob. 12PPCh. 13.3 - Write the equilibrium expression for each of the...Ch. 13.3 - Prob. 14PPCh. 13.3 - Prob. 15PPCh. 13.3 - Prob. 16PPCh. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - What is the numerical value of Kc for the...Ch. 13.3 - Prob. 21PPCh. 13.3 - Identify each of the following as a homogeneous or...Ch. 13.3 - Prob. 23PPCh. 13.3 - Prob. 24PPCh. 13.3 - Prob. 25PPCh. 13.3 - What is the numerical value of Kc for the...Ch. 13.4 - Prob. 27PPCh. 13.4 - Prob. 28PPCh. 13.4 - Indicate whether each of the following equilibrium...Ch. 13.4 - Indicale whether each of the following equilibrium...Ch. 13.4 - Prob. 31PPCh. 13.4 - The numerical value of the equilibrium constant,...Ch. 13.4 - Prob. 33PPCh. 13.4 - The numerical value of the equilibrium constant,...Ch. 13.5 - In the lower atmosphere, oxygen is converted to...Ch. 13.5 - Prob. 36PPCh. 13.5 - Hydrogen chloride can be made by reacting hydrogen...Ch. 13.5 - When heated, carbon monoxide reacts with water to...Ch. 13.5 - Use the following equation for the equilibrium of...Ch. 13.5 - Use the following equation for the equilibrium of...Ch. 13.5 - Prob. 41PPCh. 13.5 - Prob. 42PPCh. 13.6 - For each of the following slightly soluble ionic...Ch. 13.6 - For each of the following slightly soluble ionic...Ch. 13.6 - Prob. 45PPCh. 13.6 - Prob. 46PPCh. 13.6 - A saturated solution of silver carbonate, Ag2CO3 ,...Ch. 13.6 - Prob. 48PPCh. 13.6 - Calculate the molar solubility, S , of CuI if it...Ch. 13.6 - Calculate the molar solubility, S , of SnS if it...Ch. 13.6 - The CO2 level in the atmosphere has increased over...Ch. 13.6 - Prob. 52PPCh. 13 - Write the equilibrium expression for each of the...Ch. 13 - Write the equilibrium expression for each of the...Ch. 13 - Prob. 55UTCCh. 13 - Would the equilibrium constant, Ke , for the...Ch. 13 - Prob. 57UTCCh. 13 - Prob. 58UTCCh. 13 - Prob. 59APPCh. 13 - Prob. 60APPCh. 13 - For each of the following reactions, indicate if...Ch. 13 - For each of the following reactions, indicate if...Ch. 13 - Consider the reaction: (13.3) 2NH3(g)N2(g)+3H2(g)...Ch. 13 - Prob. 64APPCh. 13 - Prob. 65APPCh. 13 - Prob. 66APPCh. 13 - Prob. 67APPCh. 13 - According to Le Châtelier's principle, does the...Ch. 13 - Prob. 69APPCh. 13 - Prob. 70APPCh. 13 - The numerical value of the equilibrium constant,...Ch. 13 - The numerical value of the equilibrium constant,...Ch. 13 - For each of the following slightly soluble ionic...Ch. 13 - For each of the following slightly soluble ionic...Ch. 13 - Prob. 75APPCh. 13 - Prob. 76APPCh. 13 - Prob. 77APPCh. 13 - Prob. 78APPCh. 13 - What is the molar solubility, S , of CdS if it has...Ch. 13 - Prob. 80APPCh. 13 - Prob. 81CPCh. 13 - Prob. 82CPCh. 13 - Prob. 83CPCh. 13 - Indicate how each of the following will affect the...Ch. 13 - Prob. 85CPCh. 13 - Prob. 86CPCh. 13 - Prob. 87CPCh. 13 - Prob. 88CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- does it compare with the known melting and boiling point? (1) 9. The average kinetic energy of water molecules is a measure of the temperature of water. When the temperature of water remains constant the average kinetic energy of the molecules remains constant, even though the water is being heated by the Bunsen flame. So, energy is being taken in by the water, but it is not being used to increase the kinetic energy of the molecules. 9.1 What type of energy are the water molecules gaining during a phase change? (1) 9.2 Explain your reasoning (to question 9.1) with reference to the kinetic theory of matter. 10. Write the conclusion. (3) [30]arrow_forward5.9 Decomposition reactions are usually endothermic,whereas combination reactions are usually exothermic.Give a qualitative explanation for these trends.arrow_forward(8) 9. How much energy is required to heat 81.0 g of water (specific heat = 1.00 cal/g °C) at 11.0°C to steam (specific heat 0.468 cal/g°C) at 123.0°C? (Heat of vaporization of water = 540. cal/g)arrow_forward
- (12.7)How much heat is required to heat 28.0 grams of solid ice at -20.0 °C to liquid water at 98.0 °C? Use the appropriate data from below for the calculation. Cs, water = 4.184 J/g.°C Cs, steam = 2.01 J/g.°C Cs, ice = 2.09 J/g.°C Hfusion = 6.02 kJ/mol Hvaporization = 40.7 kJ/mol melting point of H₂O = 0 °C Boiling point of H₂O = 100 °C O 21.2 kJ O 22.0 kJ O 12.7 kJ O 26.1 kJarrow_forward9.38 The energy densities of various types of coal are listed below. Anthracite 35 kJ/g Bituminous 28 kJ/g Subbituminous 31 kJ/g Lignite 26 kJ/g Energy anUChe An unknown sample of one of these coals is burned in an apparatus with a calorimeter constant the kJ/C. When a 0.367-g sample is used, the temperature change is 8.75°C. Which type of coar s ae sample? dimen-arrow_forwardWhat mass of liquid water at room temperature (25°C) can be raised to its boiling point with the addition of 24 kJ of energy? (77g)arrow_forward
- Give examples of exothermic and endothermic reactions. (5 each)arrow_forward7.72 Classify each of the following as exothermic or endothermic: a. C3Hg(g) + 502(g) b. 2Na(s) + Cl,(g) c. PCI5(g) + 67 kJ → 3CO2(g) + 4H,O(g) + 2220 kJ 2NACI(s) + 819 kJ > PCI,(g) + Cl2(g)arrow_forward2. Solving an enthalpy of neutralization problem. (see Chemistry 2e, Example 5.5): a. Calculate q when 47.35 g of water is heated from 22.6 C to 31.1 C. cal 9 = m CAT q= (47.35) (8.5) (4.186)arrow_forward
- 10.105 Complete and balance each of the following: (10.7) a. ZnCO3(s) + H,SO,(aq) b. Al(s) + HBr(aq)arrow_forwardWhat is the enthalpy change when 0.1 moles of ethanol undergoes complete combustion? The molar enthalpy for the combustion of ethanol is -1211 kJ/mol. Is the reaction exothermic or endothermic? (1:3)arrow_forwardA coffee cup calorimeter contains 25.0 g water at 23.8 C. A 5.00 g sample of an unknown metal at an initial temperature of 78.3 C was dropped into the calorimeter. The final temperature of mixture was 46.3 C. Calculate the specific heat of the metal. The specific heat of water is (4.184)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY