
Organic Chemistry (8th Edition)
8th Edition
ISBN: 9780134042282
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 45P
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them:

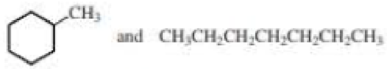
- a. CH3CH2CH2OH and CH3CH3OCH3


- b. cis-2-butene and trans-2-butene

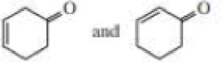
- c. i CH3CH2CH=CHCH3 and CH3CH2C=CCH3

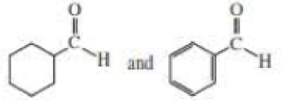

NOTE TO THE STUDENT
• There are additional spectroscopy problems in the Study Guide and Solution Manual.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
8. A strong signal in infrared spectroscopy indicates that a molecule matches the emitted
electromagnetic radiation and reports a high transmittance.
True
False
9. The signals observed from the C-C bond in an alkene will report at a higher wavenumber than
the C-C bond in an alkyne.
True
False
10. The electronegativity difference present in a dipole moment within a bond is directly
proportional to the electromagnetic field produced.
True
False
1. which spectroscopy tool would be the best to distinguish a sample of 1,2,2-trichloropropane from 1,1,2-triclopropane a) HNMR b) mass spectrometry c) infrared spectroscopy
2. Which C4H,Br isomers give rise to the two spectra shown below?
b.
8
C.
8
7
7
6
5
5
34
4
8 (ppm)
33
32 PPM
4
8 (ppm)
2H
t
I
2H
d
3
ند
3
2H 2H
quint sext
NH
2
1H
m
2
6H
d
I
C
3H
1.0 PPM
0
0
Chapter 13 Solutions
Organic Chemistry (8th Edition)
Ch. 13.1 - Which of the following fragments produced in a...Ch. 13.2 - What distinguishes the mass spectrum of...Ch. 13.2 - What is the most likely m/z value for the base...Ch. 13.3 - Prob. 5PCh. 13.3 - a. Suggest possible molecular formulas for a...Ch. 13.3 - If a compound has a molecular ion with an...Ch. 13.3 - Identify the hydrocarbon that has a molecular ion...Ch. 13.4 - Predict the relative intensities of the molecular...Ch. 13.5 - Which molecular formula has an exact molecular...Ch. 13.5 - Prob. 11P
Ch. 13.6 - Sketch the mass spectrum expected for...Ch. 13.6 - The mass spectra of 1-methoxybutane,...Ch. 13.6 - Primary alcohols have a strong peak at m/z = 31....Ch. 13.6 - Identify the ketones responsible for the mass...Ch. 13.6 - Prob. 16PCh. 13.6 - Using curved arrows, show the principal fragments...Ch. 13.6 - The reaction of (Z)-2-pentene with water and a...Ch. 13.9 - a. Which is higher in energy: electromagnetic...Ch. 13.9 - Prob. 20PCh. 13.13 - Prob. 21PCh. 13.14 - Which occur at a larger wavenumber: a. the C O...Ch. 13.14 - Prob. 23PCh. 13.14 - Prob. 24PCh. 13.14 - Rank the following compounds from highest...Ch. 13.14 - Which shows an O H stretch at a larger...Ch. 13.16 - Prob. 27PCh. 13.16 - a. An oxygen-containing compound shows an...Ch. 13.16 - Prob. 29PCh. 13.16 - For each of the following pair of compounds, name...Ch. 13.17 - Which of the following compounds has a vibration...Ch. 13.17 - Prob. 32PCh. 13.18 - A compound with molecular formula C4H6O gives the...Ch. 13.20 - Prob. 34PCh. 13.20 - Prob. 35PCh. 13.21 - Predict the max of the following compound:Ch. 13.21 - Prob. 37PCh. 13.23 - a. At pH = 7 one of the ions shown here is purple...Ch. 13.23 - Prob. 39PCh. 13.23 - Prob. 40PCh. 13 - In the mass spectrum of the following compounds,...Ch. 13 - Prob. 42PCh. 13 - Draw structures for a saturated hydrocarbon that...Ch. 13 - Rank the following compounds in order of...Ch. 13 - For each of the following pairs of compounds,...Ch. 13 - a. How could you use IR spectroscopy to determine...Ch. 13 - Assuming that the force constant is approximately...Ch. 13 - Norlutin and Enovid are ketones that suppress so...Ch. 13 - In the following boxes, list the types of bonds...Ch. 13 - A mass spectrum shows significant peaks at m/z. =...Ch. 13 - Prob. 51PCh. 13 - Prob. 52PCh. 13 - Prob. 53PCh. 13 - The IR spectrum of a compound with molecular...Ch. 13 - Rank the following compounds from highest...Ch. 13 - Rank the following compounds from highest...Ch. 13 - What peaks in their mass spectra can be used to...Ch. 13 - Prob. 58PCh. 13 - Which one of the following five compounds produced...Ch. 13 - Prob. 60PCh. 13 - Each of the IR spectra shown below is accompanied...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - How can IR spectroscopy distinguish between...Ch. 13 - Prob. 65PCh. 13 - Prob. 66PCh. 13 - Give approximate wavenumbers for the major...Ch. 13 - Prob. 68PCh. 13 - Which one of the following live compounds produced...Ch. 13 - Phenolphthalein is an acid-base indicator. In...Ch. 13 - Prob. 71PCh. 13 - How can you use UV spectroscopy to distinguish...Ch. 13 - Prob. 73PCh. 13 - The IR and mass spectra for three different...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 25. Match a structure from the list below to the following IR spectra. Place the letter of the structure in the blank below the spectrum. a. benzoic acid, C6H5COOH b. methanol, CH3OH c. benzaldehyde, C6H5CHO d. phenol, C6H5OH e. methyl acetate, CH3COOCH3 100 D 4100 LOD 50 HIT-NO-2237 |SCORE- ( Spectrum 1 D 3000 3347 B 1116 62 3336 1030 4 662 58 8 2946 18 2033 22 2622 77 2046 84 1460 47 |HIT-NO=1060 |SCORE= ( Spectrum 2 4000 1000 3229 4 2484 68 9048 15 3028 18 2962 29 2837 98 39 2725 41 2699 46 1933 57 1847 62 1711 64 1606 26 1598 10 1669 67 SOBS-NO-3302 m 1632 66 1501 14 3474 7 1372 23 43 1936 1315 47 1293 60 2008 SDBS-NO=554 HAVENUERI p 2000 1234 15 1169 31 1153 25 1072 27 1024 49 889 41 881 60 826 36 812 23 754 8 691 6 617 44 IR-NIDA-63354 : LIQUID FILM HAVENUISERI 555 18 602 20 1380 1000 IR-NIDA-29806 : LIQUID FILM mummum 1500 300 1000 500arrow_forwardA. Match the following IR spectra with their respective molecules methyl chloride (CH3Cl),methyl bromide (CH3Br), and methyl iodide (CH3I). Explain the answerS.B. Indicate the positions of C—H stretch, H—C—H bend, and other major vibrational modes inall spectra.C. Calculate the number of vibrational modes in methyl chloride.arrow_forward4. For each example below, draw at least onc possīble isomer that is consistent with the molecular formula and the associated IR spectrum. Page 3 a. MW 82, CaH1O be 48 20 1000 b. MW 116, Coll2O2 4000 2540 SL LOB2 9262 2042arrow_forward
- you are careful about what is being measured. A. B. C. Match the spectrum to the compound. All types of spectrum have been included here so make sure a. u a. C4) a. b. Br CH₂CH3 Br CH3 You observed an optical rotation, [a] of 0°. Based on this information, which of the following is the correct reaction. CH₂OH CH,00 12 b. my Micrometers NEST Chenedry Webtock ps/wkbook ist govidhemsby) OCH3 CH3 d. E Q OH OCH3 Olli. Which of the following IR spectrum is aspirin? C. 海股法 b. گار OH OCH3 L Oliti OH ww IWA CH₂CH3 P 201 dan moy Yox (192) C miruoste 101 10) A toplomarrow_forwardorganic chemistry II please label the important peaks for me and explain whyarrow_forwardChemistry 1.Predict the IR signal in the 1-bromo-3-ethylbenzene. (Approximate wave number for each Functional groups) 2.Predict the 1HNMR signal in the 1-bromo-3-ethylbenzene. (No of signals, splitting pattern of signals, integration, etc)arrow_forward
- B. Directions: Assign the IR spectra to the corresponding structures below. Cite the pertinent peaks that led to your assignment. A. Acetanilide B. Cyclohexauone C. Propionic acid N CH3 H3C HO, D. Heptanal E Ethyl acetate CH3 H, H3C CHaarrow_forwardYour lab partner sends you a draft report summarising the luminescence measurements you both carried out on compounds A. The report describes the fluorescence of the molecule with a peak at 600 nm, absorption peak at 405 nm, and phosphorescence peak at 550 nm. What is your view on this information?arrow_forwardMatch each step with the atomic spectroscopy that it occurs in. Each answer may be used once, more than once, or not at all. A photon is absorbed by the atom. A. AES and AFS A photon is emitted from the atom. The atom is excited by light. The atom is excited by thermal energy. An greater number of detected photons indicates greater amount of the analyte present. B. AES only C. AFS only D. AAS and AFS E. AAS and AES F. AAS, AES and AFS A lower number of detected photons indicates a greater amount G. AAS only of analyte present. The analyte atom is in the gas phase. The light absorbed and/or emitted is in the visible or UV range.arrow_forward
- Explain why these are the correct spectra for the molecules. Identify a specific absorption band which identifies each characteristic functional group of the molecule chosen.arrow_forward11. Predict the number of signals expected in the 13C spectrum of a. 1-bromo-2-chlorobenzene b. 1-bromo-4-chlorobenzenearrow_forward6-Infrared spectroscopy provides valuable information about molecular weight. B) melting point. C) conjugation. 7-Which of the following is not an IR vibrational mode? stretching B scissoring C rocking D rolling A) A 8- Which of these molecules best corresponds to the IR spectrum below? O • H₂C. m m m m b) m m но OH c) 3300 2900 2800 1465 1450 1375 HO 9-A strong signal at 1700 cm-1 in an IR spectrum indicates the presence of a(n) A) C) carbonyl D) amine D) functional groups. alcohol B) ether 10-Deduce the structure of an unknown compound with molecular formula CsH12O using information given by its infrared spectrum. Intensity (peak): Frequency (cm¹¹): d E wagging B- •) nammm 1000 HO OCH, X 11-Which of the following bonds would be expected to have the highest frequency= double bondarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY