
Chemistry: The Central Science (14th Edition)
14th Edition
ISBN: 9780134414232
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 31E
- Would you expect stearic acid, CH3 (CH2)16COOH, to be more soluble in water or in carbon tetrachloride?
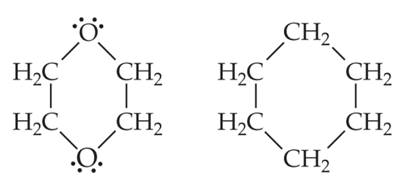
- Which would you expect to be more soluble in water, cyclohexane or dioxane?

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the
product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15%
glycerin by weight.
If the original charge is 500 kg, evaluate;
e. The ratio of sucrose to water in the original charge (wt/wt).
f. Moles of CO2 evolved.
g. Maximum possible amount of ethanol that could be formed.
h. Conversion efficiency.
i. Per cent excess of excess reactant.
Reactions:
Inversion reaction: C12H22O11 + H2O →2C6H12O6
Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2
Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3
Show work. don't give Ai generated solution. How many carbons and hydrogens are in the structure?
13. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the
molecule depicted below.
Bond B
2°C. +2°C. cleavage
Bond A
•CH3 + 26.← Cleavage
2°C. +
Bond C
+3°C•
CH3 2C
Cleavage
E
2°C. 26.
weakest bond
Intact molecule
Strongest 3°C 20.
Gund
Largest
argest
a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in
appropriate boxes.
C
Weakest
bond
A
Produces
Most
Bond
Strongest
Bond
Strongest Gund
produces least stable
radicals
Weakest
Stable radical
b. (4pts) Consider the relative stability of all cleavage products that form when bonds A,
B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B,
and C are all carbon radicals.
i. Which ONE cleavage product is the most stable? A condensed or bond line
representation is fine.
13°C. formed in
bound C
cleavage
ii. Which ONE cleavage product is the least stable? A condensed or bond line
representation is fine.
• CH3
methyl radical
Formed in Gund A Cleavage
c.…
Chapter 13 Solutions
Chemistry: The Central Science (14th Edition)
Ch. 13.3 - Prob. 13.1.1PECh. 13.3 - Prob. 13.1.2PECh. 13.3 - Prob. 13.2.1PECh. 13.3 - Prob. 13.2.2PECh. 13.4 - Prob. 13.3.1PECh. 13.4 - Prob. 13.3.2PECh. 13.4 - Prob. 13.4.1PECh. 13.4 - Prob. 13.4.2PECh. 13.4 - Prob. 13.5.1PECh. 13.4 - Prob. 13.5.2PE
Ch. 13.4 - Prob. 13.6.1PECh. 13.4 - Prob. 13.6.2PECh. 13.5 - Prob. 13.7.1PECh. 13.5 - Prob. 13.7.2PECh. 13.5 - Which aqueous solution will have the lowest...Ch. 13.5 - Prob. 13.8.2PECh. 13.5 - Prob. 13.9.1PECh. 13.5 - Prob. 13.9.2PECh. 13.5 - Prob. 13.10.1PECh. 13.5 - Practice Exercise 2
Camphor (C10 H16 O) melts at...Ch. 13.5 - Prob. 13.11.1PECh. 13.5 - Prob. 13.11.2PECh. 13 - Prob. 1DECh. 13 - Rank the contents of the following containers in...Ch. 13 - This figure shows the interaction of a cation with...Ch. 13 - Consider two ionic solids, both composed of singly...Ch. 13 - Which two statements about gas mixtures are true?...Ch. 13 - Prob. 5ECh. 13 - 13.6 If you compare the solubilities of the noble...Ch. 13 - Prob. 7ECh. 13 - Prob. 8ECh. 13 - Prob. 9ECh. 13 - Prob. 10ECh. 13 - Suppose you had a balloon made of some highly...Ch. 13 - Prob. 12ECh. 13 - Indicate whether each statement is true or false:...Ch. 13 - Indicate whether each statement is true or false:...Ch. 13 - Indicate the type of solute-solvent interaction...Ch. 13 - Indicate the principal type of solute-solvent...Ch. 13 - An ionic compound has a very negative H soln in...Ch. 13 - When ammonium chloride dissolves in water, the...Ch. 13 - Prob. 19ECh. 13 - Prob. 20ECh. 13 - Prob. 21ECh. 13 - KBr is relatively soluble in water, yet its...Ch. 13 - The solubility of Cr (NO3)3 . 9 H2O in water is...Ch. 13 - The solubility of MnSO4 . H2 O in water at 20 C is...Ch. 13 - Prob. 25ECh. 13 - Prob. 26ECh. 13 - Prob. 27ECh. 13 - Prob. 28ECh. 13 - Prob. 29ECh. 13 - Prob. 30ECh. 13 - Would you expect stearic acid, CH3 (CH2)16COOH, to...Ch. 13 - Prob. 32ECh. 13 - Prob. 33ECh. 13 - Prob. 34ECh. 13 - Indicate whether each statement is true or false:...Ch. 13 - 13.36 Indicate whether each statement is true or...Ch. 13 - The Henry’s law constant for helium gas in water...Ch. 13 - Prob. 38ECh. 13 - Prob. 39ECh. 13 - Prob. 40ECh. 13 - Prob. 41ECh. 13 - Prob. 42ECh. 13 - 13.43 Calculate the morality of the following...Ch. 13 - Prob. 44ECh. 13 - Calculate the molality of each of the following...Ch. 13 - (a) What is the molality of a solution formed by...Ch. 13 - Prob. 47ECh. 13 - Prob. 48ECh. 13 - Prob. 49ECh. 13 - The density of toluene (C7H8) is 0.867 g\mL, and...Ch. 13 - Calculate the number of moles of solute present in...Ch. 13 - Calculate the number of moles of solute present in...Ch. 13 - Prob. 53ECh. 13 - Describe how you would prepare each of the...Ch. 13 - Commercial aqueous nitric acid has a density of...Ch. 13 - Prob. 56ECh. 13 - Prob. 57ECh. 13 - Prob. 58ECh. 13 - Prob. 59ECh. 13 - Prob. 60ECh. 13 - Prob. 61ECh. 13 - Prob. 62ECh. 13 - Consider two solutions, one formed by adding 10 g...Ch. 13 - Prob. 64ECh. 13 - Prob. 65ECh. 13 - (a) Calculate the vapor pressure of water above a...Ch. 13 - Prob. 67ECh. 13 - At 20 oC, the vapor pressure of benzene (C6 H6) is...Ch. 13 - Prob. 69ECh. 13 - Prob. 70ECh. 13 - Prob. 71ECh. 13 - Prob. 72ECh. 13 - Using data from Table 13.3, calculate the freezing...Ch. 13 - Prob. 74ECh. 13 - Prob. 75ECh. 13 - Prob. 76ECh. 13 - Prob. 77ECh. 13 - Prob. 78ECh. 13 - Prob. 79ECh. 13 - Lauryl alcohol is obtained from coconut oil and is...Ch. 13 - Prob. 81ECh. 13 - Prob. 82ECh. 13 - The osmotic pressure of a 0.010 M aqueous solution...Ch. 13 - Based on the given data in Table 13.4, which...Ch. 13 - (a) Do colloids made only of gases exist? Why or...Ch. 13 - Prob. 86ECh. 13 - An “emulsifying agent” is a compound that helps...Ch. 13 - Aerosols are important components of the...Ch. 13 - Prob. 89ECh. 13 - Soaps consist of compounds such as sodium state,...Ch. 13 - Prob. 91AECh. 13 - Prob. 92AECh. 13 - Most fish need at least 4 ppm dissolved O2 in...Ch. 13 - The presence of the radioactive gas radon (Rn) in...Ch. 13 - Prob. 95AECh. 13 - Prob. 96AECh. 13 - The maximum allowable concentration of lead in...Ch. 13 - Prob. 98AECh. 13 - Prob. 99AECh. 13 - Prob. 100AECh. 13 - Prob. 101AECh. 13 - The normal boiling point of ethanol, is 78.4 0C....Ch. 13 - Prob. 103AECh. 13 - Carbon disulfide (CS2) boils at 46.30 o C and has...Ch. 13 - Prob. 105AECh. 13 - Prob. 106IECh. 13 - At ordinary body temperature (37 o C), the...Ch. 13 - Prob. 108IECh. 13 - Prob. 109IECh. 13 - Prob. 110IECh. 13 - Prob. 111IECh. 13 - Prob. 112IECh. 13 - At 35 o C the vapor pressure of acetone, (CH3)2CO,...Ch. 13 - Prob. 114IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardHi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forward. (11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B 2°C. +2°C. < cleavage Bond A • CH3 + 26. t cleavage 2°C• +3°C• Bond C Cleavage CH3 ZC '2°C. 26. E Strongest 3°C. 2C. Gund Largest BDE weakest bond In that molecule a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest C bond Produces A Weakest Bond Most Strongest Bond Stable radical Strongest Gund produces least stable radicals b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. 人 8°C. formed in bound C cleavage ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. methyl radical •CH3 formed in bund A Cleavagearrow_forward
- Which carbocation is more stable?arrow_forwardAre the products of the given reaction correct? Why or why not?arrow_forwardThe question below asks why the products shown are NOT the correct products. I asked this already, and the person explained why those are the correct products, as opposed to what we would think should be the correct products. That's the opposite of what the question was asking. Why are they not the correct products? A reaction mechanism for how we arrive at the correct products is requested ("using key intermediates"). In other words, why is HCl added to the terminal alkene rather than the internal alkene?arrow_forward
- My question is whether HI adds to both double bonds, and if it doesn't, why not?arrow_forwardStrain Energy for Alkanes Interaction / Compound kJ/mol kcal/mol H: H eclipsing 4.0 1.0 H: CH3 eclipsing 5.8 1.4 CH3 CH3 eclipsing 11.0 2.6 gauche butane 3.8 0.9 cyclopropane 115 27.5 cyclobutane 110 26.3 cyclopentane 26.0 6.2 cycloheptane 26.2 6.3 cyclooctane 40.5 9.7 (Calculate your answer to the nearest 0.1 energy unit, and be sure to specify units, kJ/mol or kcal/mol. The answer is case sensitive.) H. H Previous Nextarrow_forwardA certain half-reaction has a standard reduction potential Ered +1.26 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that must provide at least 1.10 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box.. Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. yes, there is a minimum. 1 red Πν no minimum Oyes, there is a maximum. 0 E red Dv By using the information in the ALEKS…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY