
Problems 13.43 through 13.54 require parameter values for the Wilson or NRTL equation for liquid-phase activity coefficients. Table 13.10 gives parameter values for both equations. Antoine equations for vapor pressure are given in Table B.2, Appendix B.
13.44. For one of the binary systems listed in Table 13.10. based on Eq. (13.19) and the Wilson equation, prepare a Gy diagram for P = 101.33 kPa.
Table 13.10: Parameter Values for the Wilson and NRTL Equations
Parameters a12, a21, b12 and b21 have units of cal+mol-1 and V1 and V2 have units of cm3+ mol-1
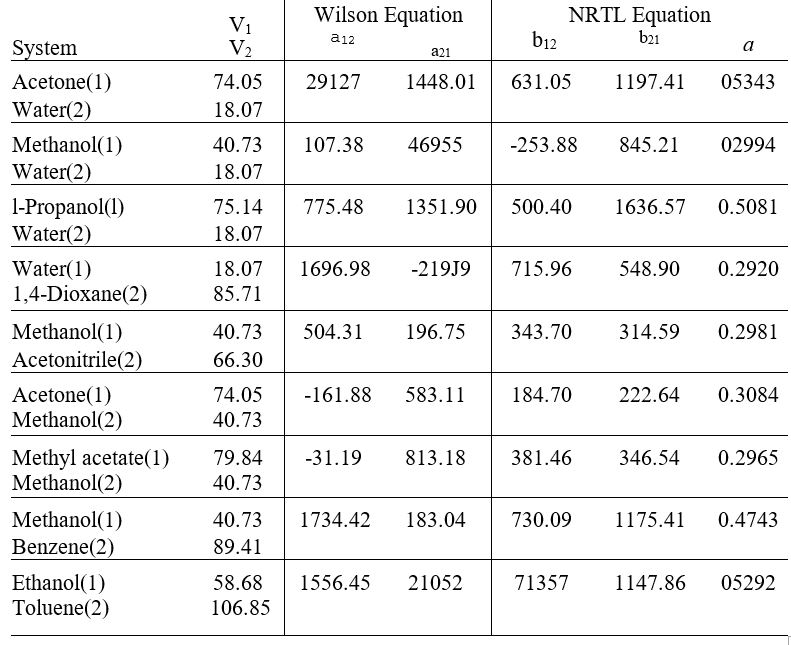
Values arc those recommended by Gmehling el al., Vapor-Liquid Equilibrium Data Collection, Chemistry Data Scries, vol. 1. parts la. lb. 2c. and 2c. DECHEM A. Franklurt/Main. 1981-1988.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Introduction to Chemical Engineering Thermodynamics
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





