
(a)
Interpretation:
The parameter values for the Margules equation which provide the best fit of GE/RTto the given data are to be determined. Also, a Pxydiagram is to be prepared and compared with the experimental data.
Concept Introduction:
Equation 13.19 to be used for Modified Raoult’s law is:
yiP=xiγiPisat ...... (1)
The formula to calculate the value of GE/RTfor a binary system is:
GERT=x1lnγ1+x2lnγ2 ...... (2)
Margules equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
GERT=(A12x1+A21x2)x1x2 ...... (3)
Here, A12 and A21are the temperature dependent parameters, specific for a system.
The equations used to calculate lnγ1 and lnγ2are:
lnγ1=x22[A12+2(A21−A12)x1]lnγ2=x21[A21+2(A12−A21)x2] ...... (4)
(a)
Answer to Problem 13.32P
The parameter values for the Margules equation which provide the best fit of GE/RTto the given data are:
A12=0.6828A21=0.4753
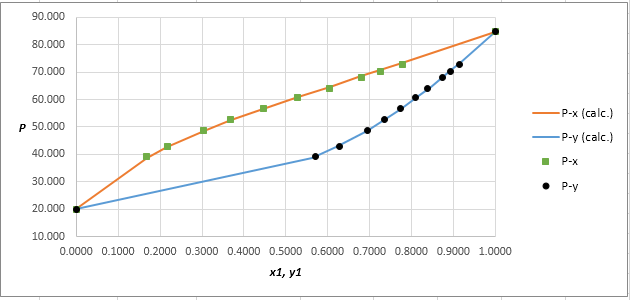
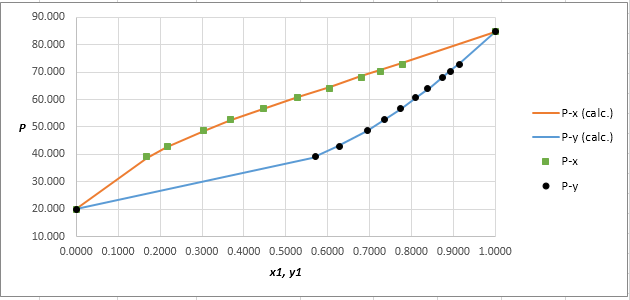
The P−x−ydiagram for the binary system containing methanol (1)/water (2) at 333.15 Kfor both calculated as well as experimental values are shown below. The values do not deviate by considerable extent from each other.
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at 333.15 Kis:
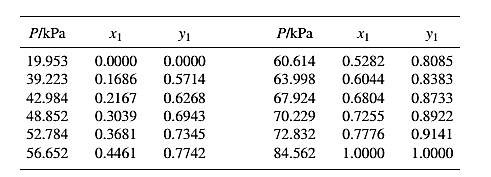
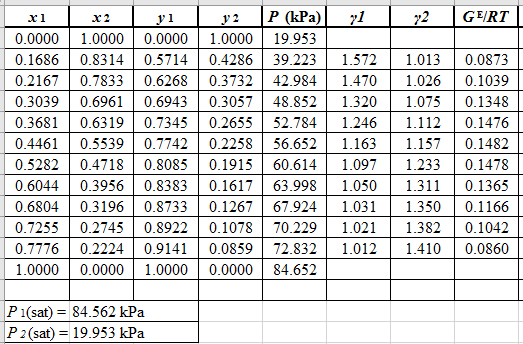
From the given data, first calculate the value of x2, and y2and tabulating then in excel spreadsheet as shown below:
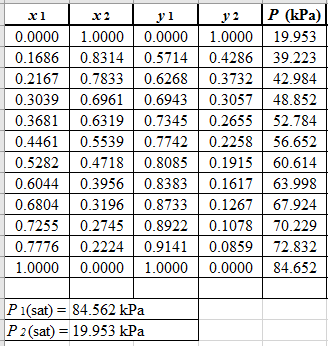
Now, use equation (1) for the Modifies Raoult’s law and calculate the value of γ1 and γ2using the below mentioned formula and tabulate it in the excel spreadsheet.
γ1=y1Px1P1satγ2=y2Px2P2sat
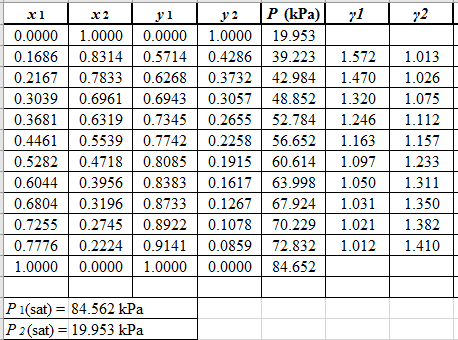
Use the equation (2) to calculate the value of GE/RTfor every value of x1and tabulate it in the excel spreadsheet as shown below:
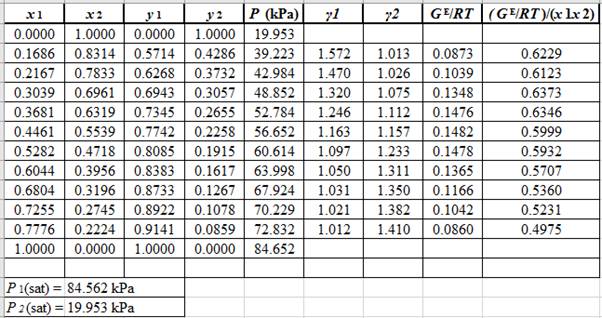
Rewrite equation (3) so that the equation becomes linear in x1as:
(GE/RTx1x2)=(A12x1+A21(1−x1))(GE/RTx1x2)=(A21−A12)x1+A12 ...... (5)
Now, calculate the values of (GE/RTx1x2)as shown below:
Plot the graph of (GE/RTx1x2)versus x1and using the excel tool as:
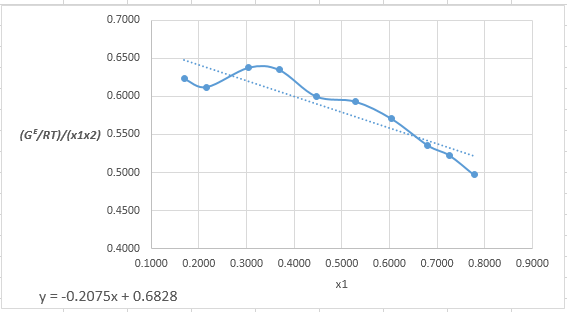
The equation that fits the plot is:
(GE/RTx1x2)=−0.2075x1+0.6828
Compare it with equation (5) so that the values of A12 and A21will be:
A12=0.6828A21=0.4753
According to the above correlation for GE/RTand the values of A12 and A21, the correlations for lnγ1 and lnγ2using the equation set (4) will be:
ln(γ1)calc.=x22[0.6828+2(0.4753−0.6828)x1](γ1)calc.=exp(x22[0.6828−0.415x1])ln(γ2)calc.=x21[0.4753+2(0.6828−0.4753)x2](γ2)calc.=exp(x21[0.4753+0.415x2])
Now, using the above relations, calculate the values of γ1 and γ2for each value of x1 and x2and tabulate the data in the excel spreadsheet as:
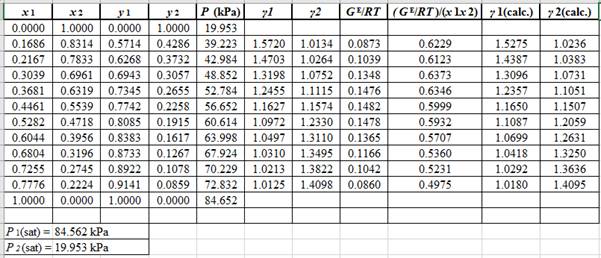
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of x1 and x2using the calculated values of γ1 and γ2as:
(P)calc.=x1(γ1)calc.P1sat+x2(γ2)calc.P2sat
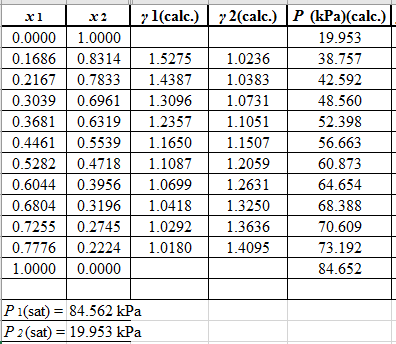
Now, use the below mentioned formula to calculate the value of y1for each of the calculated value of Pas:
(y1)calc.=x1(γ1)calc.P1sat(P)calc.
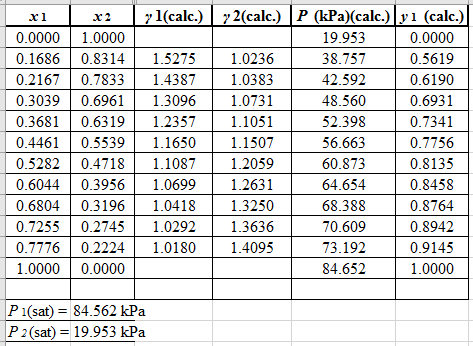
Using the tools of the excel, plot the graph of (P−x)calc., (P−y)calc., P−x, and P−yand mark labels as shown below:
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
RMS=√n∑i=1(Pi−(Pi)calc.)2nHere, n is number of entries.RMS=0.3989
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
(b)
Interpretation:
The parameter values for the van Laar equation which provide the best fit of GE/RTto the given data are to be determined. Also, a P−x−ydiagram is to be prepared and compared with the experimental data.
Concept Introduction:
Equation 13.19 to be used for Modified Raoult’s law is:
yiP=xiγiPisat ...... (1)
The formula to calculate the value of GE/RTfor a binary system is:
GERT=x1lnγ1+x2lnγ2 ...... (2)
Van Laar equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
GERT=x1x2A12A21(A12x1+A21x2) ...... (6)
Here, A12 and A21are the temperature dependent parameters, specific for a system.
The equations used to calculate lnγ1 and lnγ2from van Laar equation are:
lnγ1=A12(1+A12x1A21x2)−2lnγ2=A21(1+A21x2A12x1)−2 ...... (7)
(b)
Answer to Problem 13.32P
The parameter values for the van Laar equation which provide the best fit of GE/RTto the given data are:
A12=0.705A21=0.485
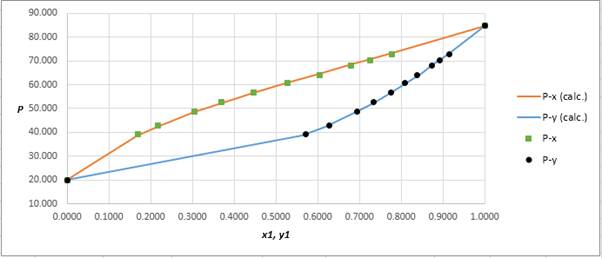
The P−x−ydiagram for the binary system containing methanol(1)/water(2) at 333.15 Kfor both calculated as well as experimental values are shown below. The values do not deviate by considerable extent from each other.
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at 333.15 Kis:
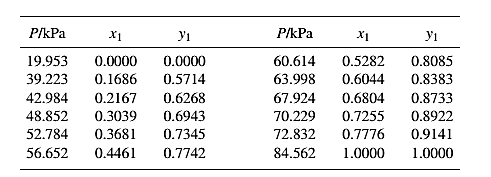
From the given data, first calculate the value of x2, and y2and tabulating then in excel spreadsheet as shown below:
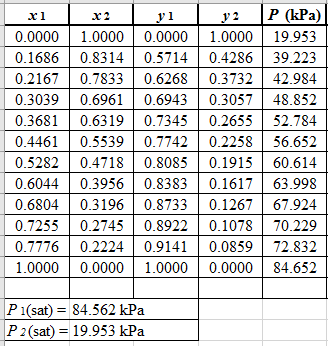
Now, use equation (1) for the Modifies Raoult’s law and calculate the value of γ1 and γ2using the below mentioned formula and tabulate it in the excel spreadsheet.
γ1=y1Px1P1satγ2=y2Px2P2sat
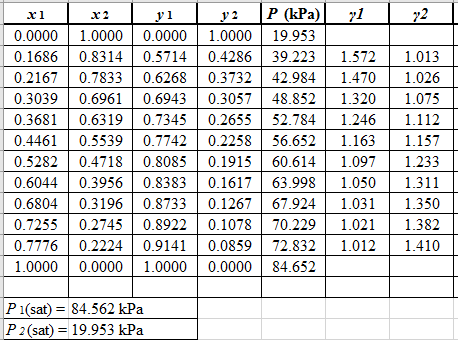
Use the equation (2) to calculate the value of GE/RTfor every value of x1and tabulate it in the excel spreadsheet as shown below:
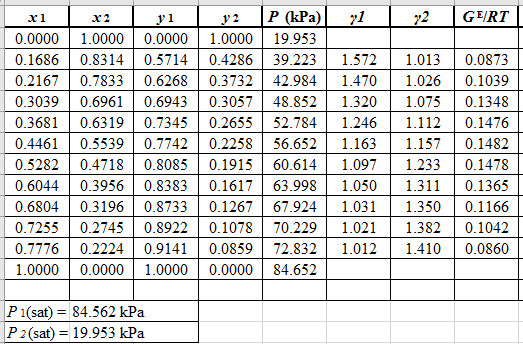
Rewrite equation (6) so that the equation becomes linear in x1as:
(GE/RTx1x2)=A12A21(A12x1+A21x2)(x1x2GE/RT)=(A12x1+A21x2)A12A21=A12x1A12A21+A21x2A12A21(x1x2GE/RT)=x1A21+1−x1A12(x1x2GE/RT)=(1A21−1A12)x1+1A12 ...... (8)
Now, calculate the values of (x1x2GE/RT)as shown below:
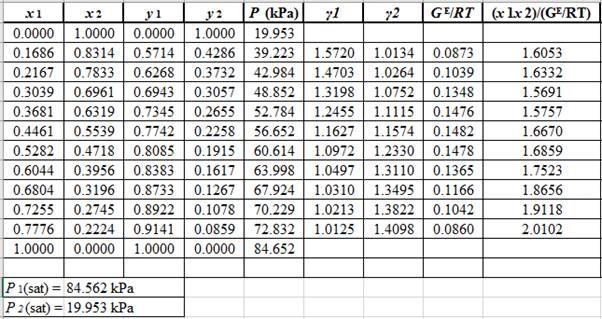
Plot the graph of (x1x2GE/RT)versus x1and using the excel tool as:
The equation that fits the plot is:
(x1x2GE/RT)=0.6414x1+1.4185
Compare it with equation (8) so that the values of A12 and A21will be:
intercept=1.4185=1A12A12=11.4185=0.705slope=0.6414=1A21−1A120.6414=1A21−10.705A21=10.6414+10.705=0.485
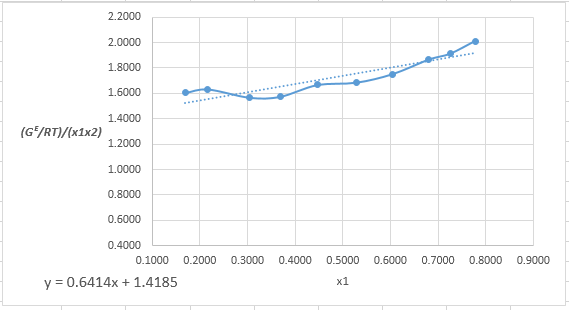
According to the above correlation for GE/RTand the values of A12 and A21, the correlations for lnγ1 and lnγ2using the equation set (7) will be:
ln(γ1)calc.=A12(1+A12x1A21x2)−2(γ1)calc.=exp[0.705(1+0.705x10.485x2)−2](γ1)calc.=exp[0.705(1+1.454x1x2)−2]ln(γ2)calc.=A21(1+A21x2A12x1)−2(γ2)calc.=exp[0.485(1+0.485x20.705x1)−2](γ2)calc.=exp[0.485(1+0.688x2x1)−2]
Now, using the above relations, calculate the values of γ1 and γ2for each value of x1 and x2and tabulate the data in the excel spreadsheet as:
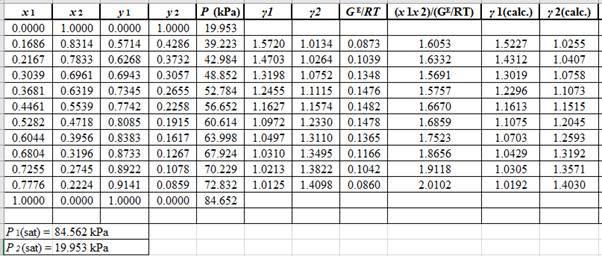
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of x1 and x2using the calculated values of γ1 and γ2as:
(P)calc.=x1(γ1)calc.P1sat+x2(γ2)calc.P2sat
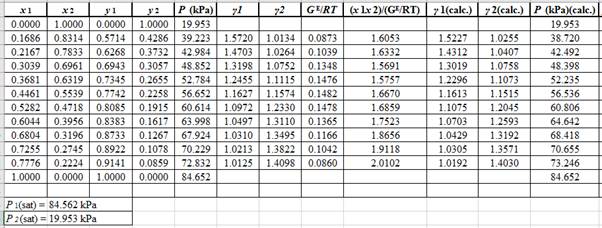
Now, use the below mentioned formula to calculate the value of y1for each of the calculated value of Pas:
(y1)calc.=x1(γ1)calc.P1sat(P)calc.
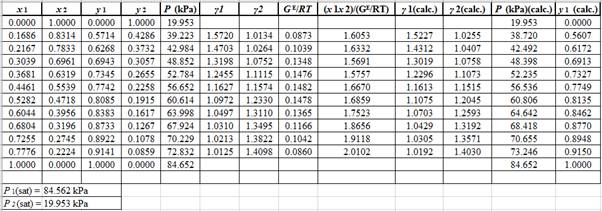
Using the tools of the excel, plot the graph of (P−x)calc., (P−y)calc., P−x, and P−yand mark labels as shown below:
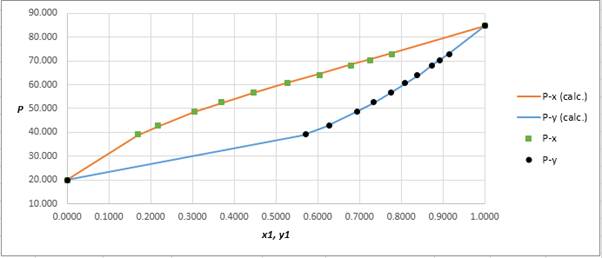
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
RMS=√n∑i=1(Pi−(Pi)calc.)2nHere, n is number of entries.RMS=0.454
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
(c)
Interpretation:
The parameter values for the Wilson equation which provide the best fit of GE/RTto the given data are to be determined. Also, a P−x−ydiagram is to be prepared and compared with the experimental data.
Concept Introduction:
Equation 13.19 to be used for Modified Raoult’s law is:
yiP=xiγiPisat ...... (1)
The formula to calculate the value of GE/RTfor a binary system is:
GERT=x1lnγ1+x2lnγ2 ...... (2)
Wilson equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
GERT=−x1ln(x1+x2Λ12)−x2ln(x2−x1Λ21) ...... (9)
Here, Λ12 and Λ21are the temperature dependent parameters, specific for a system.
The equations used to calculate lnγ1 and lnγ2from Wilson equation are:
lnγ1=−ln(x1+x2Λ12)+x2(Λ12x1+x2Λ12−Λ21x2+x1Λ21)lnγ2=−ln(x2+x1Λ21)+x1(Λ12x1+x2Λ12−Λ21x2+x1Λ21) ...... (10)
(c)
Answer to Problem 13.32P
The parameter values for the Wilson equation which provide the best fit of GE/RTto the given data are:
Λ12=0.476Λ21=1.026
The P−x−ydiagram for the binary system containing methanol(1)/water(2) at 333.15 Kfor both calculated as well as experimental values are shown below. The values deviate by considerable extent from each other.
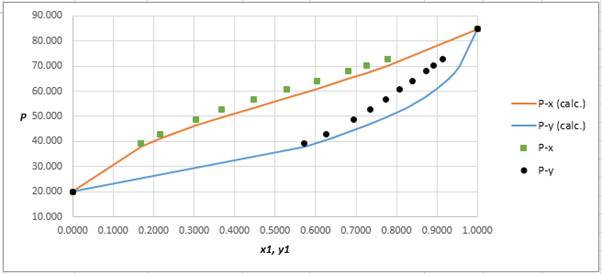
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at 333.15 Kis:
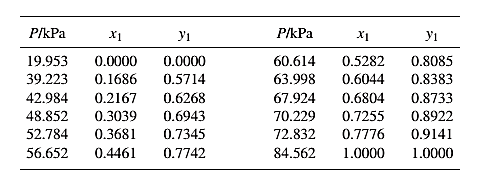
From the given data, first calculate the value of x2, and y2and tabulating then in excel spreadsheet as shown below:
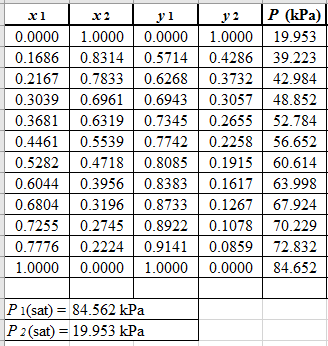
Now, use equation (1) for the Modifies Raoult’s law and calculate the value of γ1 and γ2using the below mentioned formula and tabulate it in the excel spreadsheet.
γ1=y1Px1P1satγ2=y2Px2P2sat
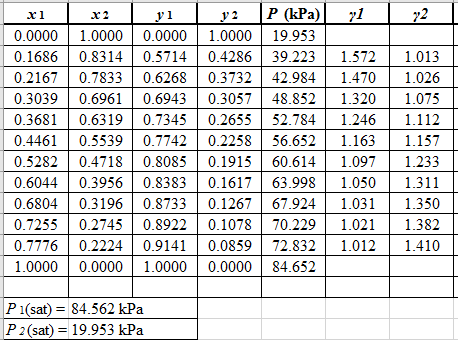
Use the equation (2) to calculate the value of GE/RTfor every value of x1and tabulate it in the excel spreadsheet as shown below:
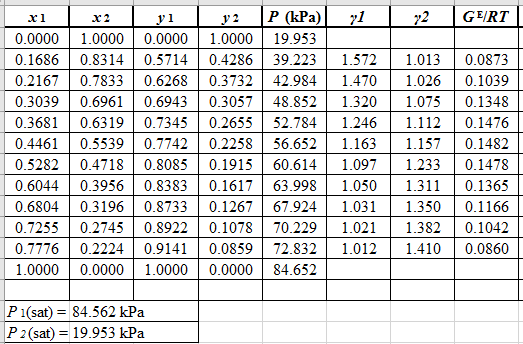
Now, use the method of the non-linear least square and fit the GE/RTdata into equation (9). First guess a value for Λ12 and Λ21 as 0.5 and 1.0respectively, then use it to calculate GE/RTfor each value of x1 . Then find the sum of the squared errors and minimize this value to get the value of the parameters Λ12 and Λ21as:
Λ12=0.476Λ21=1.026
Use the values of Λ12 and Λ21and the correlations in equation set (10) to calculate the values of γ1 and γ2 as:
Now, using the above relations, calculate the values of γ1 and γ2for each value of x1 and x2and tabulate the data in the excel spreadsheet as:
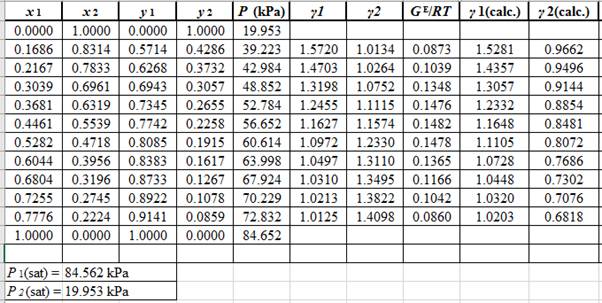
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of x1 and x2using the calculated values of γ1 and γ2as:
(P)calc.=x1(γ1)calc.P1sat+x2(γ2)calc.P2sat
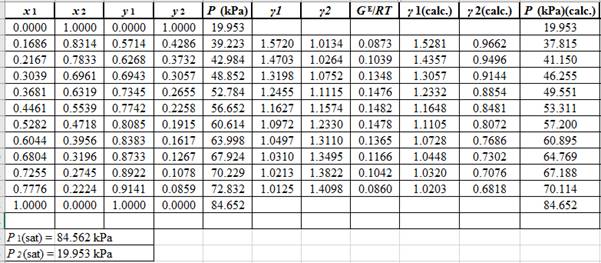
Now, use the below mentioned formula to calculate the value of y1for each of the calculated value of Pas:
(y1)calc.=x1(γ1)calc.P1sat(P)calc.
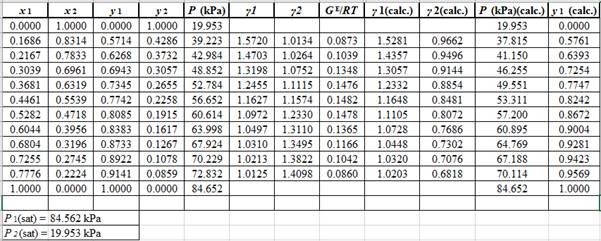
Using the tools of the excel, plot the graph of (P−x)calc., (P−y)calc., P−x, and P−yand mark labels as shown below:
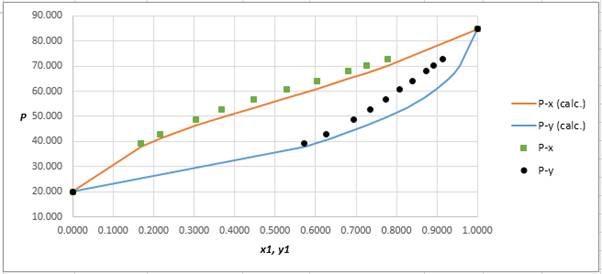
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
RMS=√n∑i=1(Pi−(Pi)calc.)2nHere, n is number of entries.RMS=2.86
Since, the RMS value is considerable, the experimental and calculated value of pressure deviate from each other by a considerable measure.
(d)
Interpretation:
The parameter values for the Margules equation which provide the best fit of P−x1data using Barker’s method data are to be determined. Also, a diagram is to be prepared to show the residuals δP and δy1which are plotted versus x1 .
Concept Introduction:
Equation 13.19 to be used for Modified Raoult’s law is:
yiP=xiγiPisat ...... (1)
Margules equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
GERT=(A12x1+A21x2)x1x2 ...... (3)
Here, A12 and A21are the temperature dependent parameters, specific for a system.
The equations used to calculate lnγ1 and lnγ2are:
lnγ1=x22[A12+2(A21−A12)x1]lnγ2=x21[A21+2(A12−A21)x2] ...... (4)
(d)
Answer to Problem 13.32P
The parameter values for the Margules equation which provide the best fit of P−x1data using Barker’s method data are:
A12=0.758A21=0.435
The plot residuals δP and δy1 versus x1on same graph is:
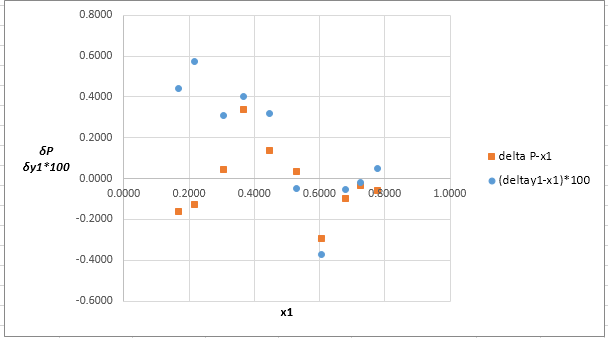
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at 333.15 Kis:
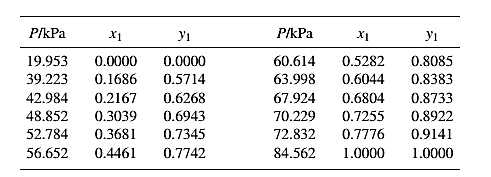
Barker’s Method is the method of determining the parameters by non-linear least squares.
Guess an initial value of A12 and A21 as 0.5 and 1.0respectively, then use it to determine the value of γ1 and γ2using equation (4) for each value of x1 .
Now, calculate the value of (P)calc.for each value of x1using equation (1) and γ1 and γ2 .
Then find the sum of the squared errors (SSE) using the below mentioned formula and minimize this value to get the value of the parameters A12 and A21as:
SSE=n∑i=1(Pi−(Pi)calc.)2(Here, n is the number of entries)A12=0.758A21=0.435
Using the equation set (4) and the parameter values, deduce the relation for γ1 and γ2 as:
ln(γ1)calc.=x22[0.758+2(0.435−0.758)x1](γ1)calc.=exp(x22[0.758−0.646x1])ln(γ2)calc.=x21[0.435+2(0.758−0.453)x2](γ2)calc.=exp(x21[0.435+0.646x2])
Now, using the above relations, calculate the values of γ1 and γ2for each value of x1 and x2and tabulate the data in the excel spreadsheet as:
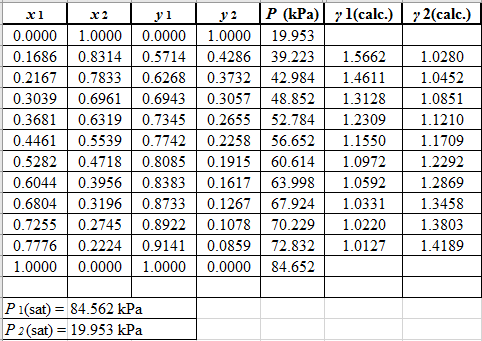
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of x1 and x2using the calculated values of γ1 and γ2as:
(P)calc.=x1(γ1)calc.P1sat+x2(γ2)calc.P2sat
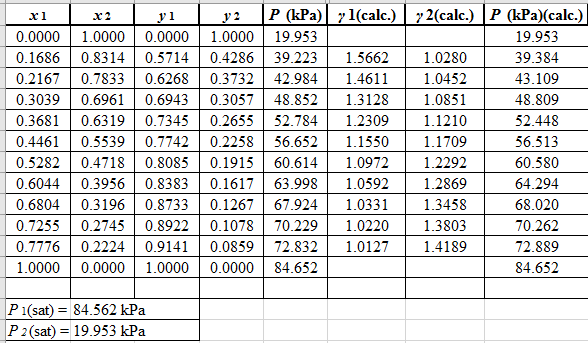
Now, use the below mentioned formula to calculate the value of y1for each of the calculated value of Pas:
(y1)calc.=x1(γ1)calc.P1sat(P)calc.
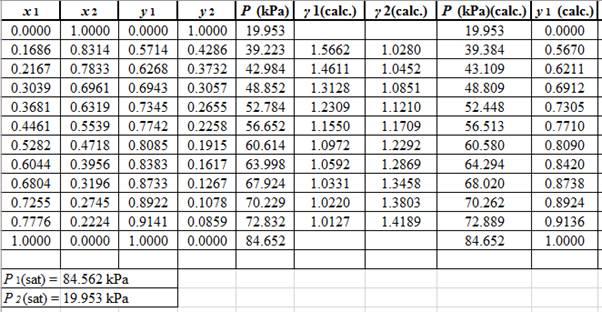
Using the tools of the excel, plot the graph of (P−x)calc., (P−y)calc., P−x, and P−yand mark labels as shown below:
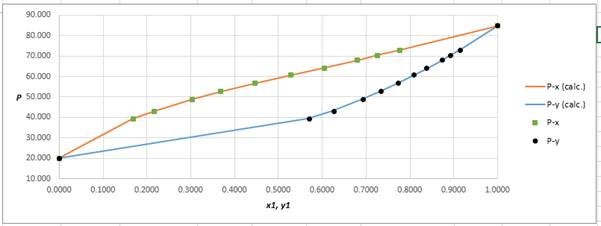
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
RMS=√n∑i=1(Pi−(Pi)calc.)2nHere, n is number of entries.RMS=0.1667
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
Now, calculate the residuals δP and δy1as shown. Also, calculate δy1×100as δy1is very small and is difficult to plot on same graph as δP .
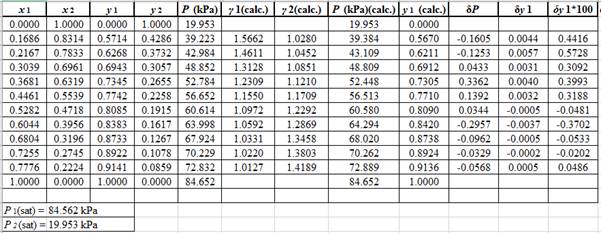
Plot these residuals against x1on same graph as:
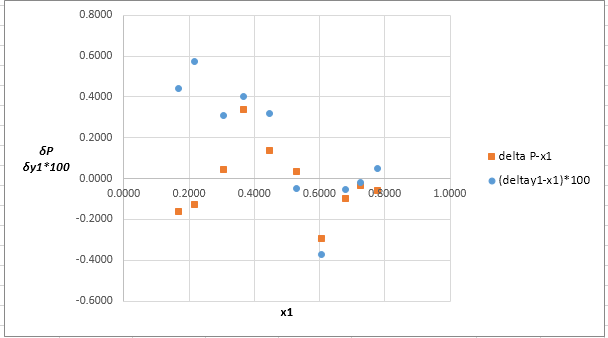
(e)
Interpretation:
The parameter values for the van Laar equation which provide the best fit of P−x1data using Barker’s method data are to be determined. Also, a diagram is to be prepared to show the residuals δP and δy1which are plotted versus x1 .
Concept Introduction:
Equation 13.19 to be used for Modified Raoult’s law is:
yiP=xiγiPisat ...... (1)
Van Laar equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
GERT=x1x2A12A21(A12x1+A21x2) ...... (6)
Here, A12 and A21are the temperature dependent parameters, specific for a system.
The equations used to calculate lnγ1 and lnγ2from van Laar equation are:
lnγ1=A12(1+A12x1A21x2)−2lnγ2=A21(1+A21x2A12x1)−2 ...... (7)
(e)
Answer to Problem 13.32P
The parameter values for the van Laar equation which provide the best fit of P−x1data using Barker’s method data are:
A12=0.830A21=0.468
The plot residuals δP and δy1versus x1on same graph is:
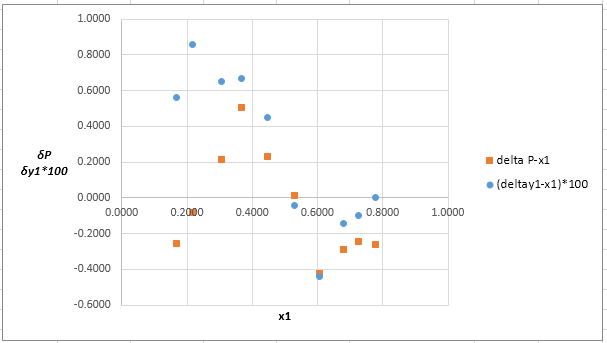
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at 333.15 Kis:
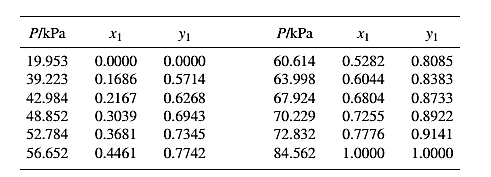
Barker’s Method is the method of determining the parameters by non-linear least squares.
Guess an initial value of A12 and A21 as 0.5 and 1.0respectively, then use it to determine the value of γ1 and γ2using equation (7) for each value of x1 .
Now, calculate the value of (P)calc.for each value of x1using equation (1) and γ1 and γ2 .
Then find the sum of the squared errors (SSE) using the below mentioned formula and minimize this value to get the value of the parameters A12 and A21as:
SSE=n∑i=1(Pi−(Pi)calc.)2(Here, n is the number of entries)A12=0.830A21=0.468
Using the equation set (7) and the parameter values, deduce the relation for γ1 and γ2 as:
ln(γ1)calc.=A12(1+A12x1A21x2)−2(γ1)calc.=exp[0.830(1+0.830x10.468x2)−2](γ1)calc.=exp[0.705(1+1.7735x1x2)−2]ln(γ2)calc.=A21(1+A21x2A12x1)−2(γ2)calc.=exp[0.468(1+0.468x20.830x1)−2](γ2)calc.=exp[0.485(1+0.5639x2x1)−2]
Now, using the above relations, calculate the values of γ1 and γ2for each value of x1 and x2and tabulate the data in the excel spreadsheet as:
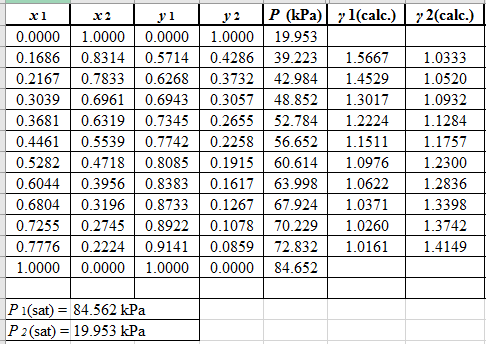
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of x1 and x2using the calculated values of γ1 and γ2as:
(P)calc.=x1(γ1)calc.P1sat+x2(γ2)calc.P2sat
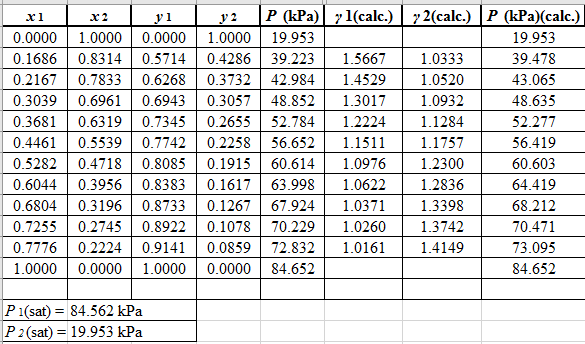
Now, use the below mentioned formula to calculate the value of y1for each of the calculated value of Pas:
(y1)calc.=x1(γ1)calc.P1sat(P)calc.
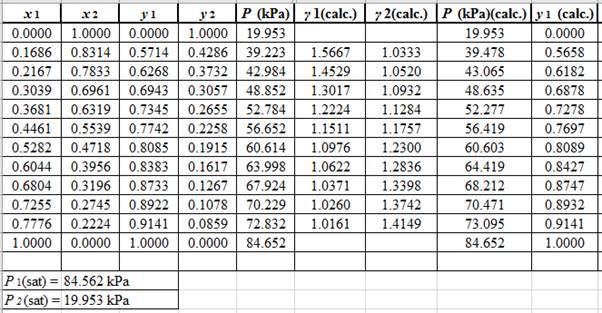
Using the tools of the excel, plot the graph of (P−x)calc., (P−y)calc., P−x, and P−yand mark labels as shown below:
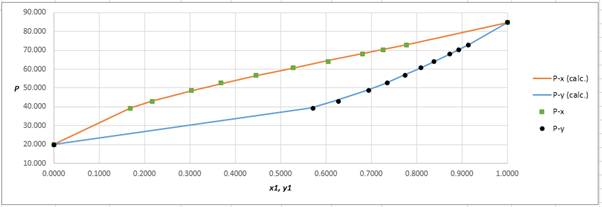
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
RMS=√n∑i=1(Pi−(Pi)calc.)2nHere, n is number of entries.RMS=0.286
Since, the RMS value is very small, the experimental and calculated value of pressure do not deviate much from each other.
Now, calculate the residuals δP and δy1as shown. Also, calculate δy1×100as δy1is very small and is difficult to plot on same graph as δP .
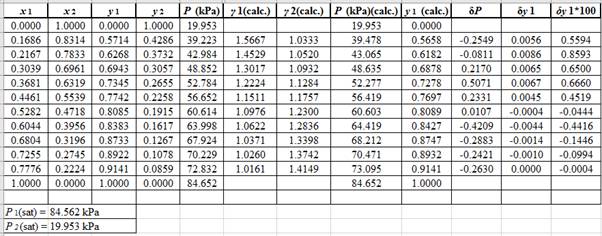
Plot these residuals against x1on same graph as:
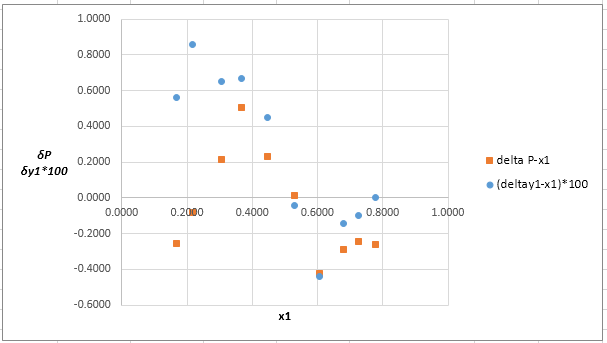
(f)
Interpretation:
The parameter values for the Wilson equation which provide the best fit of P−x1data using Barker’s method data are to be determined. Also, a diagram is to be prepared to show the residuals δP and δy1which are plotted versus x1 .
Concept Introduction:
Equation 13.19 to be used for Modified Raoult’s law is:
yiP=xiγiPisat ...... (1)
Wilson equation for excess Gibbs energy in terms of the composition of the binary system in VLE is:
GERT=−x1ln(x1+x2Λ12)−x2ln(x2−x1Λ21) ...... (9)
Here, Λ12 and Λ21are the temperature dependent parameters, specific for a system.
The equations used to calculate lnγ1 and lnγ2from Wilson equation are:
lnγ1=−ln(x1+x2Λ12)+x2(Λ12x1+x2Λ12−Λ21x2+x1Λ21)lnγ2=−ln(x2+x1Λ21)+x1(Λ12x1+x2Λ12−Λ21x2+x1Λ21) ...... (10)
(f)
Answer to Problem 13.32P
The parameter values for the Wilson equation which provide the best fit of P−x1data using Barker’s method data are:
Λ12=0.348Λ21=1.198
The plot residuals δP and δy1versus x1on same graph is:
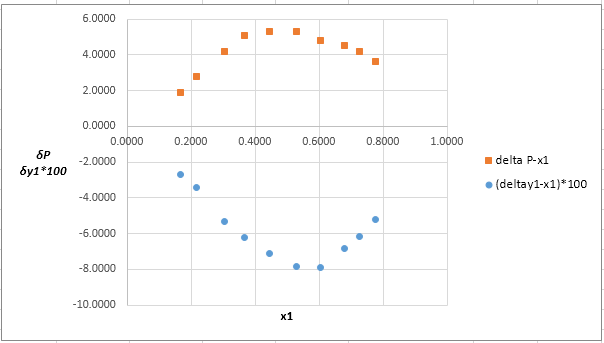
Explanation of Solution
Given information:
The set of VLE data for the binary system containing methanol(1)/water(2) at 333.15 Kis:
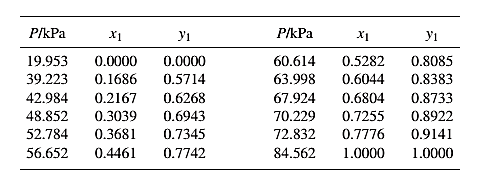
Barker’s Method is the method of determining the parameters by non-linear least squares.
Guess an initial value of A12 and A21 as 0.5 and 1.0respectively, then use it to determine the value of γ1 and γ2using equation (10) for each value of x1 .
Now, calculate the value of (P)calc.for each value of x1using equation (1) and γ1 and γ2 .
Then find the sum of the squared errors (SSE) using the below mentioned formula and minimize this value to get the value of the parameters Λ12 and Λ21as:
SSE=n∑i=1(Pi−(Pi)calc.)2(Here, n is the number of entries)Λ12=0.348Λ21=1.198
Using the equation set (10) and the parameter values, deduce the relation for γ1 and γ2 as:
Now, using the above relations, calculate the values of γ1 and γ2for each value of x1 and x2and tabulate the data in the excel spreadsheet as:
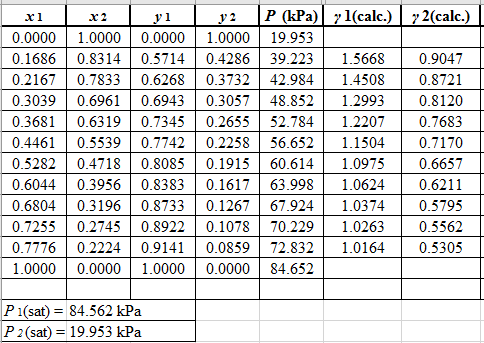
Again, use the Modified Raoult’s law equation (1) to calculate the pressure at each value of x1 and x2using the calculated values of γ1 and γ2as:
(P)calc.=x1(γ1)calc.P1sat+x2(γ2)calc.P2sat
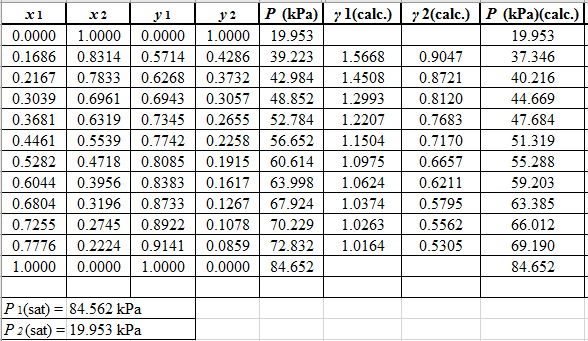
Now, use the below mentioned formula to calculate the value of y1for each of the calculated value of Pas:
(y1)calc.=x1(γ1)calc.P1sat(P)calc.
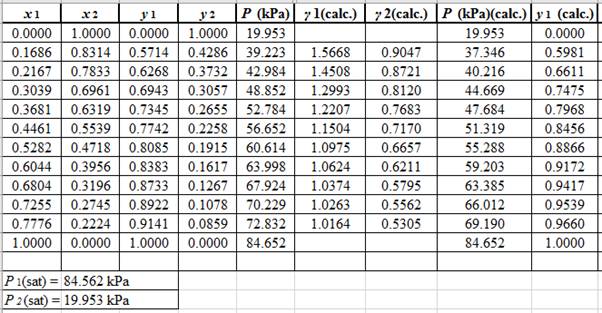
Using the tools of the excel, plot the graph of (P−x)calc., (P−y)calc., P−x, and P−yand mark labels as shown below:
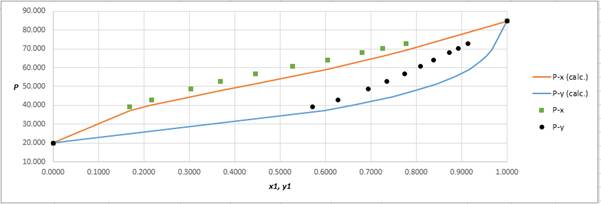
Calculate the deviation the calculated value of pressure by determining the root mean square (RMS) as shown below:
RMS=√n∑i=1(Pi−(Pi)calc.)2nHere, n is number of entries.RMS=4.314
Since, the RMS value is considerable, the experimental and calculated value of pressure deviate from each other by a considerable measure.
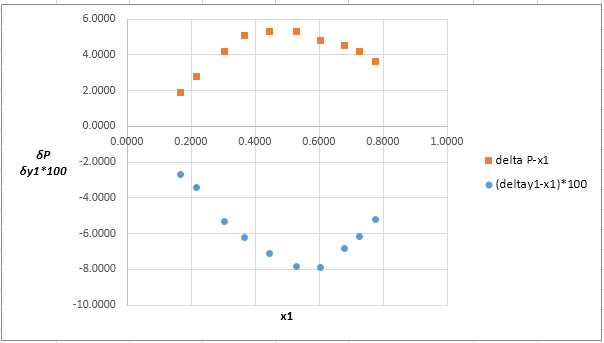
Now, calculate the residuals δP and δy1as shown. Also, calculate δy1×100as δy1is very small and is difficult to plot on same graph as δP .
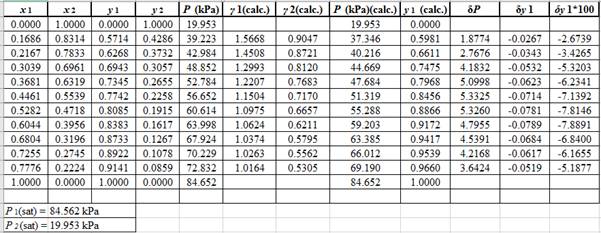
Plot these residuals against x1on same graph as:
Want to see more full solutions like this?
Chapter 13 Solutions
Introduction to Chemical Engineering Thermodynamics
- For spherical sand particles with Dp = 0.03 and ρparticles = 150 lbm / ft3 estimate the minimum fluidizing velocity for air and for water. Assume ε = 0.3. In the case of the water we must rederive Eq. 11.42, taking into account the buoyant force on the particles. Below are the provide answers. Please show all work to get to the correct answers.arrow_forwardPlease show all workarrow_forward2. A moving bed adsorption column needs to be designed to separate hydrophobic proteins from a fermentation broth. The following adsorption equilibrium data was observed in preliminary isotherm studies. The resin used was activated carbon with a porosity of 0.2. The overall mass transfer coefficient was determined to be 10 h¹, and the ratio of volumetric flow rate of broth to resin is 10. Determine the diameter of the column if the column height is limited to 2.5 m (indoor operation) with a flow rate of 20 m³/h, influent concentration of 7 g/L, and effluent concentration of 0.1 g/L. qi (mg/kg) Ci (g/L) 0.1 4.7 7.5 0.25 10.6 0.5 15.0 1.0 23.7 2.5 33.5 5.0 41.1 7.5arrow_forward
- 3. You are given a mixture of four proteins, whose properties are listed in the table below. Propose a process to purify each protein so that you end up with four solutions of pure protein. What resin would you use to bind the protein(s)? What changes to the buffer would you make to desorb the protein(s)? Contains an N-terminal His6-tag. Two 50 kDa subunits contain a non-heme Fe2+ in the active site. Protein Size (kDa) pl Specific Properties A 100 6.0 B 40 7.7 C 240 8.3 Ꭰ 225 5.5 Contains FAD redox center and an NADH binding domain. Composed of six 40-kDa subunits, each of which contains a [2Fe-2S] cluster. Composed of three subunits: 100 kDa structural subunit, 75 kDa subunit with a molybdopterin center, and 50 kDa subunit with FAD and an NADH binding domain.arrow_forwardb) Explain the key features of the Langmuir adsorption model - Drawing a diagram with empty and occupied sites. Show how new molecules would adsorb. drawing the diagram, showing free and empty sites, and their number (to use for next section) - Define the capacity and binding affinity parameters in terms of things shown on the diagram Defining the capacity and binding affinity parameters in terms of bound, free sites, and free molecules - Plot what would be a typical breakthrough curve and give an explanation approximately when breakthrough would occur plotting a typical sigmoidal breakthrough curve and saying it would certainly occur by the time capacity is used, but also could be much earlier if the affinity is lowarrow_forwardWater at 20°C flows at a steady average velocity of 5.25 m/s through a smooth pipe of diameter 5.08 cm. The flow is fully developed through the entire section of pipe. The total pipe length is 10.56 m, and there are two 90' elbows. Determine the friction coefficient and the head loss due to friction per meter length of the pipe. Control volume Prepared by Engr. Kirsten Gaarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





