
(a)
Interpretation:
The mole fraction of benzene in vapour phase, y1and constant total pressure, P at temperature 100°C is to be calculated.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(a)
Answer to Problem 13.1P
(a)
Explanation of Solution
The Raoult’s law expression for two components A and B to give the equilibrium partial pressure of the components in a binary mixture is written as follows-
and
The total pressure:
Where
The mole fraction of A in the vapor phase is given by -
Similarly, mole fraction of B in vapor phase is given by -
Now vapor pressures
Now, For benzene (1)
And For toluene (2)
Given
Now vapor pressure for benzene (1) can be calculated using Antoine equation as follows: -
Now vapor pressure for toluene (2) can be calculated using Antoine equation as follows:
Using formula
Using the formula
we find mole fraction of benzene (1) in vapor phase, 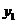
Therefore,
(b)
Interpretation:
The mole fraction of benzene in liquid phase, x1and constant total pressure, P at temperature 100°C is to be calculated.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(b)
Answer to Problem 13.1P
Explanation of Solution
The Raoult’s law expression for two components A and B to give the equilibrium partial pressure of the components in a binary mixture is written as follows-
and
The total pressure:
Where
The mole fraction of A in the vapor phase is given by -
Similarly, mole fraction of B in vapor phase is given by -
Now vapor pressures
Now, For benzene (1)
And For toluene (2)
Given
Now vapor pressure for benzene (1) can be calculated using Antoine equation as follows: -
Now vapor pressure for toluene (2) can be calculated using Antoine equation as follows:
Using the formula
we find mole fraction of benzene (1) in liquid phase, 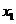
Using formula
Therefore,
(c)
Interpretation:
The mole fraction of benzene in vapor phase, y1and temperature of the solution, T at total pressure P =120 kPa is to be calculated.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(c)
Answer to Problem 13.1P
Explanation of Solution
The Raoult’s law expression for two components A and B to give the equilibrium partial pressure of the components in a binary mixture is written as follows-
and
The total pressure:
Where
The mole fraction of A in the vapor phase is given by -
Similarly, mole fraction of B in vapor phase is given by -
Now vapor pressures
Now, For benzene (1)
And For toluene (2)
Given
Now,
Using formula
Solving this equation, we find
Now vapor pressure for benzene (1) can be calculated using Antoine equation as follows: -
Now vapor pressure for toluene (2) can be calculated using Antoine equation as follows:
Using the formula
we find mole fraction of benzene (1) in vapor phase, 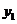
Therefore,
(d)
Interpretation:
The mole fraction of benzene in liquid phase, x1and temperature of the solution, T at total pressure P =120 kPa is to be calculated.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(d)
Answer to Problem 13.1P
Explanation of Solution
The Raoult’s law expression for two components A and B to give the equilibrium partial pressure of the components in a binary mixture is written as follows-
and
The total pressure:
Where
The mole fraction of A in the vapor phase is given by -
Similarly, mole fraction of B in vapor phase is given by -
Now vapor pressures
Now, For benzene (1)
And For toluene (2)
Given
Now,
Solving these two equations, we find
Therefore,
(e)
Interpretation:
The mole fraction of benzene in liquid phase, x1and mole fraction of benzene in vapour phase, y1at total pressure P =120 kPa and temperature T=105 °C is to be calculated.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(e)
Answer to Problem 13.1P
Explanation of Solution
The Raoult’s law expression for two components A and B to give the equilibrium partial pressure of the components in a binary mixture is written as follows-
and
The total pressure:
Where
The mole fraction of A in the vapor phase is given by -
Similarly, mole fraction of B in vapor phase is given by -
Now vapor pressures
Now, For benzene (1)
And For toluene (2)
Given
Now,
Now vapor pressure for benzene (1) can be calculated using Antoine equation as follows: -
Now vapor pressure for toluene (2) can be calculated using Antoine equation as follows:
Using formula
Using the formula
Therefore,
(f)
Interpretation:
The overall mole fraction of benzene is z1=0.33 at total pressure P =120 kPa and temperature T=105 °C is given. The mole fraction of benzene and toluene in vapor phase needs to calculated.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(f)
Answer to Problem 13.1P
Vapor fraction, V is 0.231923 and liquid fraction is 0.768077
Explanation of Solution
The Raoult’s law expression for two components A and B to give the equilibrium partial pressure of the components in a binary mixture is written as follows-
and
The total pressure:
Where
The mole fraction of A in the vapor phase is given by -
Similarly, mole fraction of B in vapor phase is given by -
Now vapor pressures
Now, For benzene (1)
And For toluene (2)
Given
Now,
Now vapor pressure for benzene (1) can be calculated using Antoine equation as follows: -
Now vapor pressure for toluene (2) can be calculated using Antoine equation as follows:
Using formula
Using the formula
Now,
Therefore, vapor fraction, V is 0.231923 and liquid fraction is 0.768077.
(g)
Interpretation:
To explain why Raoul’s law is likely to be an excellent VLE model for this system at the stated conditions.
Concept introduction:
Raoult’s Law states that the partial pressure of liquid A above the solution is equal to the mole fraction of the liquid in a solution times the partial pressure of the pure liquid. This holds for ideal solutions. An ideal solution is approached by binary solutions of molecules that have similar properties (e.g. benzene and toluene). The law is mathematically expressed as-
Where
A solution behaves ideally when
- The solute and the solvent molecules have similar sizes and similar intermolecular forces,
- The excess volume of mixing is zero, and
- The heat of mixing is zero when both the solute and the solvent are liquids
(g)
Explanation of Solution
Benzene and toluene are both non-polar. They are similar in shape and size. Therefore, one would expect little chemical interaction between the two substances. Moreover, the temperature is sign enough and the pressure is low, so it is expected to behave ideally.
Want to see more full solutions like this?
Chapter 13 Solutions
Introduction to Chemical Engineering Thermodynamics
- Q1: Consider the following transfer function G(s) 5e-s 15s +1 1. What is the study state gain 2. What is the time constant 3. What is the value of the output at the end if the input is a unit step 4. What is the output value if the input is an impulse function with amplitude equals to 3, at t=7 5. When the output will be 3.5 if the input is a unit steparrow_forwardgive me solution math not explinarrow_forwardgive me solution math not explinarrow_forward
- give me solution math not explinarrow_forwardgive me solution math not explinarrow_forwardExample (6): An evaporator is concentrating F kg/h at 311K of a 20wt% solution of NaOH to 50wt %. The saturated steam used for heating is at 399.3K. The pressure in the vapor space of the evaporator is 13.3 KPa abs. The 5:48 O Transcribed Image Text: Example (7): Determine thearrow_forward
- 14.9. A forward feed double-effect vertical evaporator, with equal heating areas in each effect, is fed with 5 kg/s of a liquor of specific heat capacity of 4.18 kJ/kg K. and with no boiling point rise, so that 50 per cent of the feed liquor is evaporated. The overall heat transfer coefficient in the second effect is 75 per cent of that in the first effect. Steam is fed at 395 K and the boiling point in the second effect is 373 K. The feed is heated by an external heater to the boiling point in the first effect. It is decided to bleed off 0.25 kg/s of vapour from the vapour line to the second effect for use in another process. If the feed is still heated to the boiling point of the first effect by external means, what will be the change in steam consumption of the evaporator unit? For the purpose of calculation, the latent heat of the vapours and of the steam may both be taken as 2230 kJ/kgarrow_forwardExample(3): It is desired to design a double effect evaporator for concentrating a certain caustic soda solution from 12.5wt% to 40wt%. The feed at 50°C enters the first evaporator at a rate of 2500kg/h. Steam at atmospheric pressure is being used for the said purpose. The second effect is operated under 600mmHg vacuum. If the overall heat transfer coefficients of the two stages are 1952 and 1220kcal/ m2.h.°C. respectively, determine the heat transfer area of each effect. The BPR will be considered and present for the both effect 5:49arrow_forwardالعنوان ose only Q Example (7): Determine the heating surface area 개 required for the production of 2.5kg/s of 50wt% NaOH solution from 15 wt% NaOH feed solution which entering at 100 oC to a single effect evaporator. The steam is available as saturated at 451.5K and the boiling point rise (boiling point evaluation) of 50wt% solution is 35K. the overall heat transfer coefficient is 2000 w/m²K. The pressure in the vapor space of the evaporator at atmospheric pressure. The solution has a specific heat of 4.18kJ/ kg.K. The enthalpy of vaporization under these condition is 2257kJ/kg Example (6): 5:48 An evaporator is concentrating F kg/h at 311K of a 20wt% solution of NaOH to 50wt %. The saturated steam used for heating is at 399.3K. The pressure in the vapor space of the evaporator is 13.3 KPa abs. The 5:48 1 J ۲/۱ ostrarrow_forward
- Example 8: 900 Kg dry solid per hour is dried in a counter current continues dryer from 0.4 to 0.04 Kg H20/Kg wet solid moisture content. The wet solid enters the dryer at 25 °C and leaves at 55 °C. Fresh air at 25 °C and 0.01Kg vapor/Kg dry air is mixed with a part of the moist air leaving the dryer and heated to a temperature of 130 °C in a finned air heater and enters the dryer with 0.025 Kg/Kg alry air. Air leaving the dryer at 85 °C and have a humidity 0.055 Kg vaper/Kg dry air. At equilibrium the wet solid weight is 908 Kg solid per hour. *=0.0088 Calculate:- Heat loss from the dryer and the rate of fresh air. Take the specific heat of the solid and moisture are 980 and 4.18J/Kg.K respectively, A. =2500 KJ/Kg. Humid heat at 0.01 Kg vap/Kg dry=1.0238 KJ/Kg. "C. Humid heat at 0.055 Kg/Kg 1.1084 KJ/Kg. "C 5:42 Oarrow_forwardQ1: From the Figure below for (=0.2 find the following 1. Rise Time 2. Time of oscillation 3. Overshoot value 4. Maximum value 5. When 1.2 which case will be? 1.6 1.4 1.2 12 1.0 |=0.8- 0.6 0.4 0.8 0.2- 0.6 0.4 0.2 1.2 = 1.0 0 2 4 6 8 10 10 t/Tarrow_forwardPlease, I need solution in detailsarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





