
(a)
Interpretation:
Mechanism for the tautomerization process that occurs under acidic conditions has to be drawn.
Concept Introduction:
Keto-enol tautomerization occurs when a hydroxyl group is present next to a double bond in presence of acid or base. Due to this the

Acid catalyzed tautomerization:
Compounds that are ketone can undergo keto-enol tautomerization in presence of acid. Tautomers are not same as resonance structures. The chemical properties of tautomers will be different. A general mechanism of keto-enol tautomerization that occur in acidic condition can be given as,
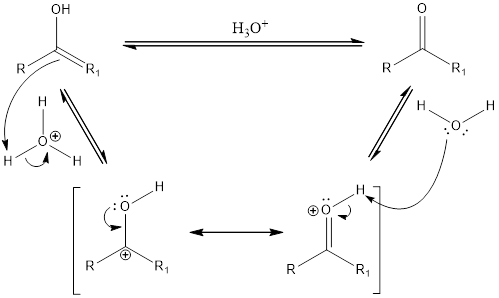
First step is the formation of carbocation. Second step is the formation of double bond between oxygen and carbon atom. The water acts as a base and abstracts a proton to form the final keto compound.
(b)
Interpretation:
Mechanism for the tautomerization process that occurs under basic conditions has to be drawn.
Concept Introduction:
Keto-enol tautomerization occurs when a hydroxyl group is present next to a double bond in presence of acid or base. Due to this the ketone will have few amount of enol also. The quantity of ketone and enol is determined only by equilibrium.

Base catalyzed tautomerization:
Compounds that are ketone can undergo keto-enol tautomerization in presence of acid. Tautomers are not same as resonance structures. The chemical properties of tautomers will be different. A general mechanism of keto-enol tautomerization that occur in basic condition can be given as,
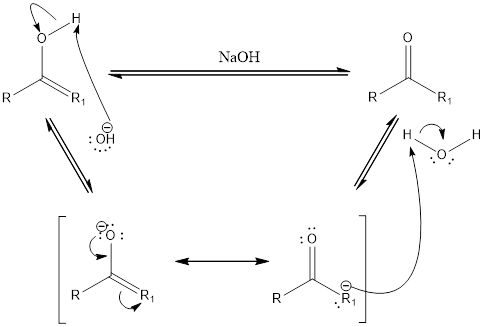
First step is the removal of proton. Second step is the formation of double bond between oxygen and carbon atom. The water acts as an acid and donates a proton to form the final keto compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry As a Second Language: First Semester Topics
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





