
Concept explainers
Interpretation:
The base shown is sufficiently strong or not to deprotonate the given alkyne has to be determined and if it is strong enough, the formed alkynide ion has to be drawn.
Concept Introduction:

In order to deprotonate a terminal alkyne, the base that is used has to be less stable than the resulting alkynide ion.
There are few factors which determine the strength of the acid and they are,
- What atom the charge is present
- Resonance
- Induction
- Orbitals
If the charge is on a more electronegative atom, then it is stabilized more. Hence, the compound will be more acidic.
If the negative charge is made to participate in resonance, then the negative charge will be stabilized. This increases the stability of the conjugate base and in turn the compound will be more acidic.
Pull of the electron density by the more electronegative atom is known as induction. The inductive effects can stabilize or destabilize the conjugate base. If the inductive effect stabilizes the conjugate base, then the compound will be acidic.
The orbital in which the negative charge is present also plays an important role in stability of the conjugate base. A negative charge on
In order to find whether the compound is more acidic or not, the first step is to remove the proton to form conjugate base. Then look for the above four factors. For the stability of bases, the first factor and the fourth factor is the most important one.
The stability order due to electronegativity can be given as shown below,
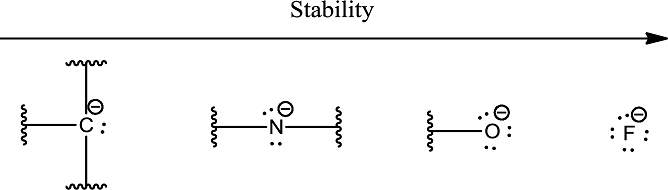
The stability order due to factor 4 is based upon the hybridization.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry As a Second Language: First Semester Topics
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





