
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Textbook Question
thumb_up100%
Chapter 12, Problem 83E
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
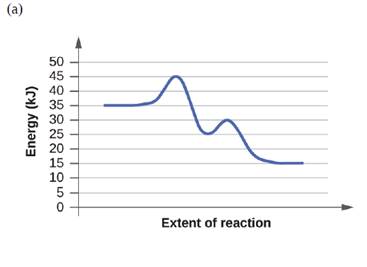
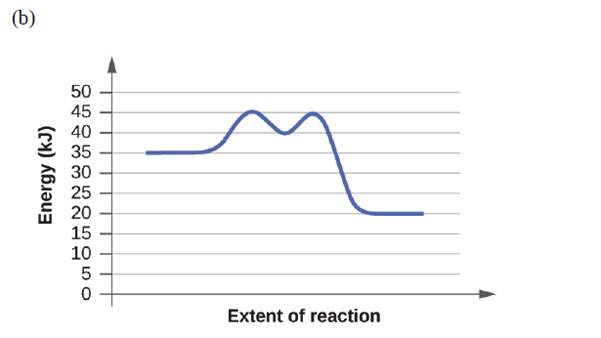
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A gas following mole compositions at
120 \deg F, 13.8 psia. N2% 2, CH
4% 79C2H6 % 19. Volume fractionn?
Please correct answer and don't used hand raiting
Order-disorder phenomenaa) do not have conductive properties.b) are cooperative.c) have few industrial implications.
Chapter 12 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 12 - What is the difference between average rate,...Ch. 12 - Ozone decomposes to oxygen according to the...Ch. 12 - In the nuclear industry, chlorine trifluoride is...Ch. 12 - A study of the rate of dimerization of C4H6 gave...Ch. 12 - A study of the rate of the reaction represented as...Ch. 12 - Consider the following reaction in aqueous...Ch. 12 - Describe the effect of each of the following on...Ch. 12 - Explain why an egg cooks move slowly in boiling...Ch. 12 - Go to the PhET Reactions and change to Angled...Ch. 12 - In the PhET Reactions tab to observe how multiple...
Ch. 12 - In the PhET Reactions under Options. (a) Leave...Ch. 12 - How do the rate of a reaction and its rate...Ch. 12 - Doubling the concentration of a reactant increases...Ch. 12 - Tripling the concentration of a reactant increases...Ch. 12 - How much and in what direction will each of the...Ch. 12 - How will each of the following affect the rate of...Ch. 12 - Regular ?ights of supersonic aircraft in the...Ch. 12 - Radioactive phosphorus is used in the study of...Ch. 12 - The rate constant for the radioactive decay of 14C...Ch. 12 - The decomposition of acetaldehyde is a second...Ch. 12 - Alcohol is removed from the bloodstream by a...Ch. 12 - Under certain conditions the decomposition of...Ch. 12 - Nitrosyl chloride, NOCI, decomposes to NO and CI2....Ch. 12 - From the following data, determine the rate...Ch. 12 - Nitrogen monoxide reacts with chlorine according...Ch. 12 - Hydrogen reacts with nitrogen monoxide to form...Ch. 12 - For the reaction AB+C, the following data were...Ch. 12 - For the reaction QW+X, the following data were...Ch. 12 - The rate constant for the ?rst-order decomposition...Ch. 12 - The annual production of HNO3 in 2013 was 60...Ch. 12 - The following data have been determined for the...Ch. 12 - Describe how graphical methods can be used to...Ch. 12 - Use the data provided to graphically determine the...Ch. 12 - Use the data provided in a graphical method to...Ch. 12 - Pure ozone decomposes slowly to oxygen, 2O33O2(g)....Ch. 12 - From the given data, use a graphical method to...Ch. 12 - What is the half-life for the first-order decay of...Ch. 12 - What is the half-life for the first-order decay of...Ch. 12 - What is the half-life for the decomposition of...Ch. 12 - What is the half-life for the decomposition of O3...Ch. 12 - The reaction of compound A to give compounds C and...Ch. 12 - The half-life of a reaction of compound A to give...Ch. 12 - Some bacteria are resistant to the antibiotic...Ch. 12 - Both technetium-99 and thallium-201 are used to...Ch. 12 - There are two molecules with the formula C3H6...Ch. 12 - Fluorine-18 is a radioactive isotope that decays...Ch. 12 - Suppose that the half-life of steroids taken by an...Ch. 12 - Recently, the skeleton of King Richard III was...Ch. 12 - Nitroglycerine is an extremely sensitive...Ch. 12 - For the past 10 years, the unsaturated hydrocarbon...Ch. 12 - Chemical reactions occur when reactants collide....Ch. 12 - When every collision between reactants leads to a...Ch. 12 - What is the activation energy of a reaction, and...Ch. 12 - Account for the relationship between the rate of a...Ch. 12 - Describe how graphical methods can be used to...Ch. 12 - How does an increase in temperature affect rate of...Ch. 12 - The rate of a certain reaction doubles for every...Ch. 12 - In an experiment, a sample of NaClO3 was 90%...Ch. 12 - The rate constant at 325 C for the decomposition...Ch. 12 - The rate constant for the decomposition of...Ch. 12 - An elevated level of the enzyme alkaline...Ch. 12 - In terms of collision theory, to which of the...Ch. 12 - Hydrogen iodide, HI, decomposes in the gas phase...Ch. 12 - The element Co exists in two oxidation states,...Ch. 12 - The hydrolysis of the sugar sucrose to the sugars...Ch. 12 - Use the PhET Reactions Single collision" tab of...Ch. 12 - Use the PhET Reactions Single collision tab of the...Ch. 12 - Why awe elementary reactions involving three or...Ch. 12 - In general, can we predict the effect of doubling...Ch. 12 - Define these terms: (a) unimolecular reaction (b)...Ch. 12 - What is the rate equation for the elementary...Ch. 12 - Given the following reactions and the...Ch. 12 - Write the rate equation for each of the following...Ch. 12 - Nitrogen (Il) oxide, NO, reacts with hydrogen, H2,...Ch. 12 - Experiments were conducted to study the rate of...Ch. 12 - The reaction of CO with CI2 gives phosgene...Ch. 12 - . Account for the increase in reaction rate...Ch. 12 - Compare the functions of homogeneous and...Ch. 12 - Consider this scenario and answer the following...Ch. 12 - For each of the following pairs of reaction...Ch. 12 - For each of the following pairs of reaction...Ch. 12 - For each of the following reaction diagrams,...Ch. 12 - For each of the following reaction diagrams,...Ch. 12 - Based on the diagrams in Exercise 12.83, which of...Ch. 12 - Based on the diagram in Exercise 12.83, which of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match the people in column A to their contribution toward the advancement of microbiology, in column B. Column ...
Microbiology: An Introduction
CAUTION Why does the presence of extinct forms and transitional features in the fossil record support the patte...
Biological Science (6th Edition)
7. Both Tim and Jan (problem 6) have a widow’s peak (see Module 9.8), but Mike has a straight hairline. What ar...
Campbell Biology: Concepts & Connections (9th Edition)
In the following diagram, the white spheres represent hydrogen atoms and the blue Sphere represent the nitrogen...
Chemistry: The Central Science (14th Edition)
1. How many cervical, thoracic, lumbar, sacral, and coccygeal vertebrae are normally present in the vertebral ...
Human Anatomy & Physiology (2nd Edition)
The bond angles in a regular polygon with n sides are equal to 180360n a. What are the bond angles in a regular...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward1:14 PM Fri 20 Dec 67% Grade 7 CBE 03/12/2024 (OOW_7D 2024-25 Ms Sunita Harikesh) Activity Hi, Nimish. When you submit this form, the owner will see your name and email address. Teams Assignments * Required Camera Calendar Files ... More Skill: Advanced or complex data representation or interpretation. Vidya lit a candle and covered it with a glass. The candle burned for some time and then went off. She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? * (1 Point) She wanted to check whether the length of the candle would affect the time for which it burns. She performed the experiment again after changing something. Which of these would be the correct experimental setup for her to use? A Longer candle; No glass C B Longer candle; Longer glass D D B Longer candle; Same glass Same candle; Longer glassarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY