
Concept explainers
Use the figure below to answer Question 4.
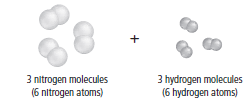
Hydrogen and nitrogen react as shown to formammonia
A. Three ammonia molecules are formed, with zeromolecules remaining.
B. Two ammonia molecules are formed, with twohydrogen molecules remaining.
C. Six ammonia molecules are formed, with zeromolecules remaining.
D. Two ammonia molecules are formed, with twonitrogen molecules remaining.
Interpretation:
True statement regarding ammonia formation must be selected from the given list.
Concept introduction:
1 mole nitrogen reacts with 3 moles hydrogen to produce 2 moles of ammonia.
Answer to Problem 4STP
Correct answer: Option D is correct.
Explanation of Solution
Reason for correct option: Option D is correct because 1 molecule of nitrogen reacts with 3 molecules of hydrogen to produce 2 molecules of ammonia. Remaining nitrogen molecule will be
Reasons for incorrect options: Option A is incorrect as 3 ammonia molecules will not be formed.
Option B is incorrect as no hydrogen molecules will be left.
Option C is incorrect as six ammonia molecules will not be formed.
Chapter 12 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Chemistry: A Molecular Approach (4th Edition)
Chemistry: A Molecular Approach
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: Structure and Properties (2nd Edition)
Chemistry: Structure and Properties
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





