
Concept explainers
Interpretation:
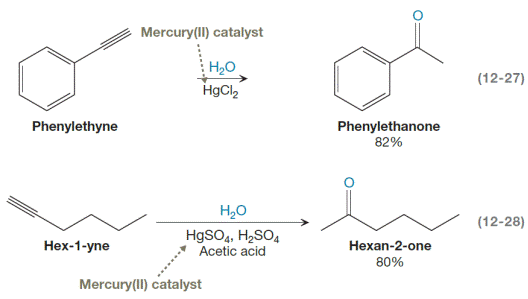
Equations 12-27 and 12-28 show the overall products of hydration of the
Concept introduction:
Alkynes can undergo Markovnikov addition of water via oxymercuration. Hydration of an alkyne leads to an initial enol, which tautomerizes into the more stable keto form. A
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Draw out a complete arrow-pushing mechanism for the following reaction. This reaction is explicitly done with heat. O NaOH, H₂O heatarrow_forwardBy taking into account electronegativity differences, draw the products formed by heterolysis of the carbon–heteroatom bond in each molecule. Classify the organic reactive intermediate as a carbocation or a carbanion.arrow_forwardComplete synthesis in 2-3 stepsarrow_forward
- Fill in the missing intermediates(two), regents(two), and the fill product in the following multi-step synthesis. Assume all reactions proceed too quickly for rearrangements to take placearrow_forwardComplete the following multi step synthesis by showing the major intermediate products and the reagents nessesary for each steparrow_forwardIn a Wittig reaction, a ketone or aldehyde reacts as an electrophile with a nucleophile called a Wittig reagent (or phosphonium ylide) to produce an alkene. The Wittig reagent is commonly synthesized first in a two-step process beginning with an alkyl halide, then reacted with the carbonyl compound. In this problem, you'll explore the mechanism of a multi-step synthesis to make an alkene using the Wittig approach.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





