
(a)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to
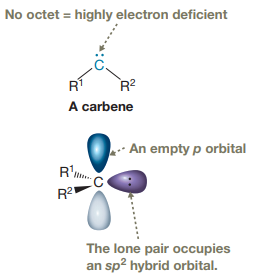
Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
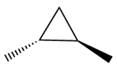
The given compound can be produced from an alkene is shown below:
Explanation of Solution
The given compound is:
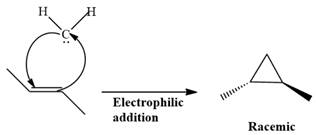
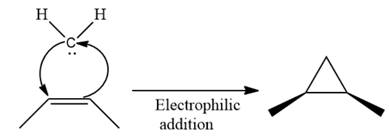
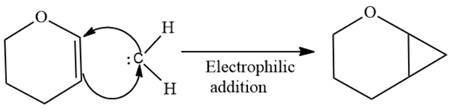
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
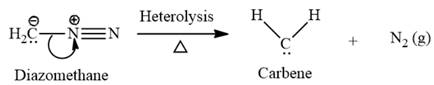
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
![]()
The complete reaction of the addition of carbene to alkene is shown below:
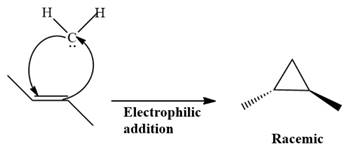
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
(b)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. In carbine, the carbon atom has two bonds and a lone pair of electrons.

Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
The given compound can be produced from an alkene is shown below:

Explanation of Solution
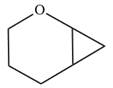
The given compound is:
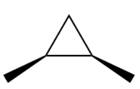
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
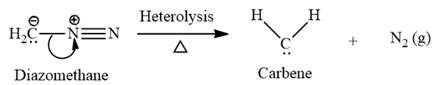
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
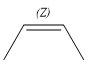
The complete reaction of the addition of carbene to alkene is shown below:
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
(c)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to alkenes form cyclopropane rings. In carbine, the carbon atom has two bonds and a lone pair of electrons.

Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
The given compound can be produced from an alkene is shown below:

Explanation of Solution
The given compound is:
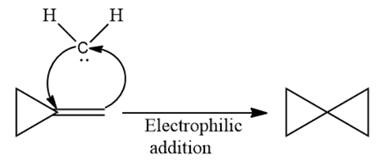
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
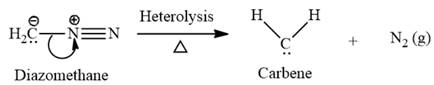
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
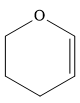
The complete reaction of the addition of carbene to alkene is shown below:
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
(d)
Interpretation:
How the given compound can be produced from an alkene is to be shown.
Concept introduction:
Electrophilic addition of carbenes to alkenes forms cyclopropane rings. In carbine, the carbon atom has two bonds and a lone pair of electrons.

Carbenes are typically highly electrophilic due to the lack of an octet and are
Answer to Problem 12.2P
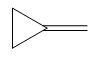
The given compound can be produced from an alkene is shown below:
Explanation of Solution
The given compound is:

Electrophilic addition of carbenes to alkenes forms cyclopropane rings. Diazomethane (
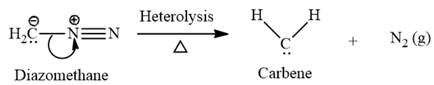
This carbene intermediate reacts with an alkene in a concerted one-step reaction to form a cyclopropyl ring. The bridge carbon atom in the cyclopropyl ring comes from the carbene while the two carbon atoms are from the original alkene. Two new
The complete reaction of the addition of carbene to alkene is shown below:
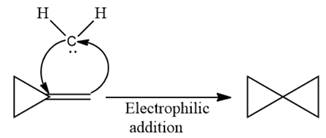
The given compound is produced via an electrophilic addition reaction of a carbene to an alkene.
Want to see more full solutions like this?
Chapter 12 Solutions
Organic Chemistry: Principles And Mechanisms
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

