
Concept explainers
(a)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(a)
Answer to Problem 10A
Explanation of Solution
The structure of water is:
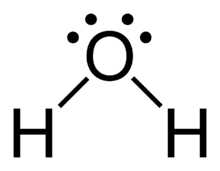
It contains two
(b)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(b)
Answer to Problem 10A
Explanation of Solution
The structure of
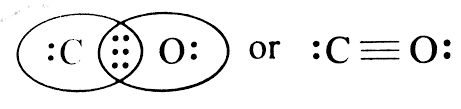
It contains one
(c)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(c)
Answer to Problem 10A
Explanation of Solution
The structure of
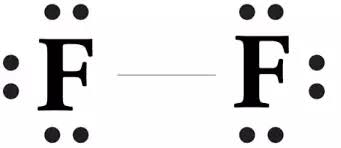
The bond is formed between same atoms. So it is no-polar bond.
(d)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(d)
Answer to Problem 10A
Explanation of Solution
The structure of
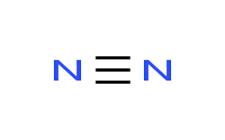
The bond is formed between same atoms. So it is no-polar bond.
Chapter 12 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





