
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 11.8, Problem 17P
Interpretation Introduction
Interpretation:
To write the mechanism for the acid-catalyzed transesterification of ethyl acetate and methanol.
Concept introduction:
Transesterification is the process of formation of a new ester molecule from the reaction of alcohol and an ester. This is like hydrolysis of ester but here nucleophile is alcohol molecule instead of
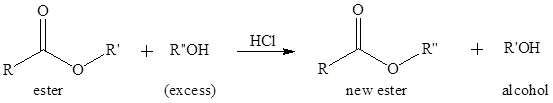
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the reaction mechanism for acetic acid production from methanol carbonylation, using a metal complex as a catalyst?
Describe a reaction mechanism that occurs to maleic acid when hydrochloric acid is added.
Describe the Concept of Acid-Catalyzed Ester Hydrolysis and Transesterification.
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write chemical reaction of the epoxide preparation by reacting cis-2-pentene with a peroxycarboxylic acid. peroxycarboxylic acidarrow_forwardwrite the mechanism for a decarboxylation and state the structural features necessary for a decarboxylation.arrow_forwardin the oxidation of cyclohexanol to cyclohexanone, what purpose does the acetic acid serve?arrow_forward
- The synthsis of isopentyl acetate is classified as which of the following reaction types? Acyl group substitution O Acyl group addition Acyl group oxidation Acyl group eliminationarrow_forwardDraw and explain the mechanism for the ammoxidation of propene which leads to the formation of acrolein.arrow_forwardDraw out the reaction mechanism for cyclohexanol to cyclohexanone. Sodium hypochlorite oxidation of an alcohol to a ketone with the product being cyclohexanone.arrow_forward
- Which is a dehydrating agent in the preparation of Pyroxylin USP? H3BO3 HCl H2SO4 HNO3arrow_forwardWhen 4-hydroxybutanoic acid is treated with an acid catalyst, it forms a lactone (a cyclic ester). Draw the structural formula of this lactone and propose a mechanism for its formationarrow_forwardList the products of the reaction of acetaldehyde and acetone with sodium ethoxide in ethanol.Explain it brieflyarrow_forward
- 2 4. Write the metabolism pathway for the following molecule via epoxidation of the aromatic ring and nucleophilic attack. CH3 NH₂ p-chloroamphetamine 2arrow_forwardIndicate the product of the reaction diethyl propanedioate 1° EtONA, EtOH 2° nitroethenearrow_forwardAcetal derivatives of aldehydes and ketones are prepared by an acid-catalyzed dehydration reaction with alcohols or diols. Using more pictures than words, draw a reaction and a mechanism that shows the formation of acetal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY