
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11.5, Problem 8P
Using the pKa values listed in Table 11.1, predict the products of the following reactions:
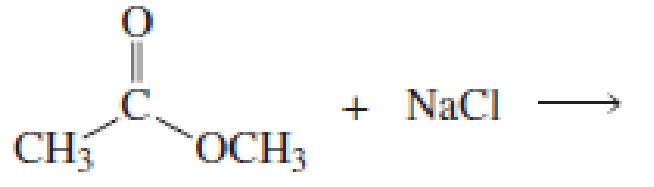
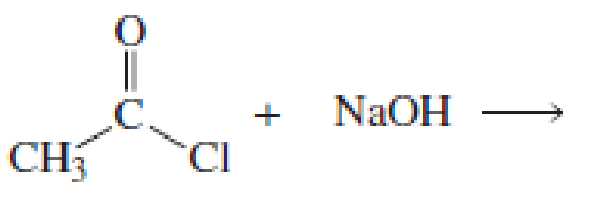
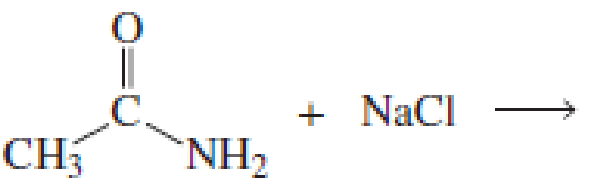
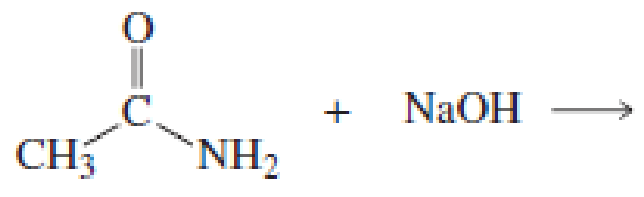
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sulfuric acid (H2SO4) is called a diprotic acid because it has two acidic protons. The pKa for the first deprotonation is -9, whereas the pKa for the second deprotonation is 2. Explain these relative acid strengths.
The normal pH range for blood plasma is 7.35–7.45. Under these conditions, would you expect the carboxyl group of lactic acid (pKa 3.08) to exist primarily as a carboxyl group or as a carboxylic anion? Explain.
Which of the following reactions favor formation of the products? (For the pKa values necessary to solve this problem, see Appendix I. Recall that the equilibrium favors formation of the weaker acid
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compound p-methoxybenzonitrile N-oxide, which has the formula CH3OC6H4CNO, reacts with itself to form a dimer—a molecule that consists of two p-methoxybenzonitrile N-oxide units connected to each other (CH3OC6H4CNO)2. The reaction can be represented as A + A → B or 2 A → B where A represents p-methoxybenzonitrile N-oxide and B represents the dimer, (CH3OC6H4CNO)2. For the reaction in carbon tetrachloride at 40 °C with an initial concentration of 0.011 M, these data were obtained: Determine the rate law for the reaction. Determine the rate constant. Determine the order of the reaction with respect to A.arrow_forwardDicarboxylic acids have two dissociation constants, one for the initial dissociation into a monoanion and one for the second dissociation into a dianion. For oxalic acid, HO2C—CO2H, the first ionization constant is pKal = 1.2 and the second ionization constant is pKa2 = 4.2. Why is the second carboxyl group far less acidic than the first?arrow_forwardwhy do these compounds show this trend in pka?arrow_forward
- Consider the following compounds that vary from nearly nonacidic to strongly acidic. Draw the conjugate bases of these compounds, and explain why the acidity increases so dramatically with substitution by nitro groups.CH4 CH3NO2 CH2(NO2)2 CH(NO2)3 pKa ≅ 50 pKa = 10.2 pKa = 3.6 pKa = 0.17arrow_forwardIs it justifiable to neglect the hydrolysis of PO43- in the calculation for Ka3? Explain your reasoning.arrow_forwardCalculate the percentage of free acid for (a) phenobarbital (it is an acid with pKa = 7.40) and (b) hexobarbital (also an acid with pKa = 8.4) at the physiological condition of pH 7.4.arrow_forward
- (a) Is 2-pyridone aromatic?(b) Use resonance forms to explain your answer to (a). Also explain why the protons atd 7.31 and d 7.26 are more deshielded than the other two (d 6.15 and d 6.57)arrow_forwardName the following compounds A and B. How could you distinguish these two molecules by using 1H NMR and IR techniques? Propose an analytical technique to determine the iron content of these compounds. Calculate the mass percentages of C and H of compound B (C: 12.01 g/mol; H: 1.008 g/mol; Fe: 55.845 g/mol).arrow_forwardHow would the pKa values of carboxylicacids, alcohols, ammonium ions 1RNH32 + , phenol, and an anilinium ion 1C6H5NH32 + change if they were determined in a solvent less polar than water?arrow_forward
- Predict the products of the following reaction: Na + Al2S3 →arrow_forwardWhat factors would result in the following equation favoring the products? Cd(NO3)2∙4H2O(s) ⟷ Cd(NO3)2(s) + 4H2O(g)arrow_forwardExplain the difference in acidity between p-methoxybenzoic acid (pKa = 4.46) and m-methoxybenzoic acid (pKa = 4.09)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
What are CHNOPS? These Chemical Elements = 98% of Life | Biology | Biochemistry; Author: Socratica;https://www.youtube.com/watch?v=w90wFlR53VM;License: Standard YouTube License, CC-BY