
Concept explainers
(a)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
A reagent is a substance used to convert one chemical compound into another.
Transesterification: Transesterification reaction is an esterification reaction of ester react with excess of alcohol in the presence of either acid or base catalyst to form a new ester. The formation of one type of ester can be transformed in to other form of esters is called esterification when reaction moves forward when we use excess of an alcohol.

(b)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Ester reaction with sodium hydroxide which goves the sodium salt and alcohol.
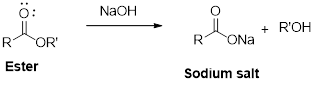
It is an example of saponification reaction.
(c)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Amination reaction: Amination is the process by which an
(d)
Interpretation:
The reagent has to be identified for the conversion of methyl propanoate to the following compounds.
Concept introduction:
Ester reaction with sodium hydroxide which gives the sodium salt and alcohol this sodium salt further reaction with dil. Hydrochloric acid gives acid.

It is an example of saponification reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- Which of the following carboxylic acid is toxic when ingested? a.Citric acid b.Malic acid c.Acetic acid d.Oxalic acid What is the general formula for Grignard reagents? a.RCOOH b.RMgX c.RX d.RCOX Oxidation of alkyl benzenes in the presence of Sulfuric acid will produce which carboxylic acid? a.Cyclohexanecarboxylic acid b.Benzoic acid c.Acrylic acid d.Fumaric acid What dicarboxylic acid contains six carbon atoms in its structure? a.Capric acid b.Pimelic acid c.Adipic acid d.Hexanoic acid Which of the following is an example of an unsaturated carboxylic acid? a.Succinic acid b.Formic acid c.Glycolic acid d.Acrylic acidarrow_forwardWhich alkyl halides form the carboxylic acids listed here after reaction with sodium cyanide followed by heating the product in an acidic aqueous solution? a. butyric acid b. isovaleric acid c. cyclohexanecarboxylic acidarrow_forward3. Give the pharmacological property of the following alkaloids. Where do they naturally occur? a. morphine b. quinine c. berberine 4. What are the chemical tests used in identifying alkaloids?arrow_forward
- 45. Which of the following compounds has the lowest water solubility? a. butanal b. heptanal c. hexanal d. pentanal 48. Which of the following compounds is the most soluble in water? a. butanal b. heptanal c. hexanal d. pentanal 56. Which of the following compounds will undergo oxidation using potassium dichromate to form a carboxylic acid? A a. A and B only b. A and C only c. B only d. C only H B o Carrow_forwardHydrolysis of the ester CH3-CH2-COO-CH2-CH2-CH3 in dilute acid, gives a. acetic acid and ethanol b. propanoic acid and ethanol c. propanoic acid and 1- propanol d. acetic acid and 1-propanolarrow_forward5. What products would result from the following processes? Write an equation for each reaction. a. 2-Methyl-2-butanol is subjected to controlled oxidation. b. 1-Propanol is heated to 140°C in the presence of sulfuric acid. c. 3-Pentanol is subjected to controlled oxidation. d. 3-Pentanol is heated to 180°C in the presence of sulfuric acid. e. 1-Hexanol is subjected to an excess of oxidizing agentarrow_forward
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardDescribe the reaction when a. Formaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid. b. Acetaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid. c. Benzaldehyde mixed with a fainty pink solution of potassium permanganate with a few drops of sulfuric acid.arrow_forwardWhich of the following compounds can react with C2H5MgBr to give 3-pentanol? a.acetone b.ethanal c.acetic acid d.ethyl formatearrow_forward
- What are the functional groups present in this antibacterial antibiotic? A. Amide, thioether, aldehyde, phenol, carboxylic acid B. Amide, thioether, ketone, amine, phenol, carboxylic acid C. Amide, thioether, ketone, phenol, carboxylic acid D. Thioether, ketone, amine, phenol, carboxylic acid A brief explanation would be highly appreciated + upvotearrow_forwardErythronolide B is the biological precursor of erythromycin, a broad-spectrum antibiotic. What functional group Erythronolide B does contain? a. b. H₂CH₂C C. H₂C 1 H₂C 2 3 4 O Amide d. Amine OH Erythronolide B Ketone Aldehyde a CH₂ b C d CH₂ OH JCH₂ 'OH OHarrow_forwardThe reactant: benzophenone & 2-propanol the product: benzopinacol & acetonearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

