
Which of the following would you expect to be more soluble in water than in benzene? (Select all that apply.)
(a)
(b)
(e)
Interpretation:
The compounds that is more soluble in water than benzene is/are to be identified.
Concept introduction:
The various kinds of interactions that bind a molecule are known as intermolecular forces. These can be dispersion, dipole–dipole, ion–dipole,and hydrogen bonding.
The solubility of a compound depends on the intermolecular forces present. Apolar solvent is soluble in apolar solvent only. A compound is soluble in water only when it is able to form hydrogen bonds with it.
Answer to Problem 1KSP
Solution: The correct answersareoption (a) and option (e).
Explanation of Solution
Reason for the correct option:
The molecule, methanol
Thus, it is soluble in water more readily. On the other hand, benzene is a nonpolar molecule. So, methanol is insoluble in benzene.
Potassium iodide
Hence, option (a) and option (e) are correct.
Reasons for the incorrect options:
Option (b) is incorrect because
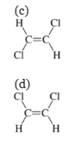
Option (c) is incorrect because trans-dichloroethene is a nonpolar molecule. It is insoluble in water. So, this option is incorrect.
Option (d) is incorrect because cis-dichloroethene is a polar molecule but it is unable to form hydrogen bonds in water. It is, therefore, insoluble in water. So, this option is incorrect.
Hence, options(c) and (d) are incorrect.
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry
- Hi!! Please provide a solution that is handwritten. Ensure all figures, reaction mechanisms (with arrows and lone pairs please!!), and structures are clearly drawn to illustrate the synthesis of the product as per the standards of a third year organic chemistry course. ****the solution must include all steps, mechanisms, and intermediate structures as required. Please hand-draw the mechanisms and structures to support your explanation. Don’t give me AI-generated diagrams or text-based explanations, no wordy explanations on how to draw the structures I need help with the exact mechanism hand drawn by you!!! I am reposting this—ensure all parts of the question are straightforward and clear or please let another expert handle it thanks!!arrow_forwardIn three dimensions, explain the concept of the velocity distribution function of particles within the kinetic theory of gases.arrow_forwardIn the kinetic theory of gases, explain the concept of the velocity distribution function of particles in space.arrow_forward
- In the kinetic theory of gases, explain the concept of the velocity distribution function of particles.arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION with its parts spread out till part (g), please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all calculations step by step EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forwardHi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part PART A AND PART B!!!!! till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward
- Hi!! Please provide a solution that is handwritten. this is an inorganic chemistry question please answer accordindly!! its just one question with parts JUST ONE QUESTION, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward8b. Explain, using key intermediates, why the above two products are formed instead of the 1,2-and 1,4- products shown in the reaction below. CIarrow_forward(5pts) Provide the complete arrow pushing mechanism for the chemical transformation depicted below Use proper curved arrow notation that explicitly illustrates all bonds being broken, and all bonds formed in the transformation. Also, be sure to include all lone pairs and formal charges on all atoms involved in the flow of electrons. CH3O H I I CH3O-H H I ① Harrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning




