
Concept explainers
The following names are incorrect, according to IUPAC rules. Draw the structural formulas and tell why each name is incorrect. Write the correct name for each compound.
a.
b.
c.
d.
(a)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
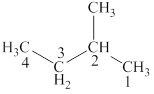
The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
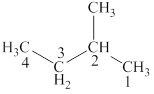
Figure 1
The structural formulas for given compound is shown in Figure 1. The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
(b)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
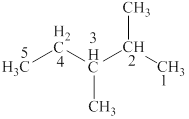
The lowest possible number is given to the carbon at which a group is attached. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is

Figure 2
The structural formulas for given compound is shown in Figure 2. The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is
(c)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
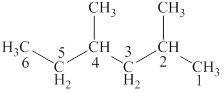
The parent chain is hexane and one methyl group each is attached to second and fourth carbon atom. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has six carbon atoms. Thus, the parent chain is hexane. The lowest possible number is given to the carbon from which a group is attached. Thus, methyl group are attached to second and fourth carbon. Thus, the correct IUPAC name of the given compound is

Figure 3
The structural formulas for given compound is shown in Figure 3. The parent chain is hexane and one methyl group each is attached to second and fourth carbon atoms. Thus, the correct IUPAC name of the given compound is
(d)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
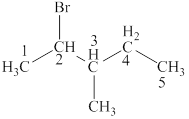
The parent chain is pentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has five carbon atoms. Thus, the parent chain is pentane. The lowest possible number is given to the carbon from which a group is attached. Thus, bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is

Figure 4
The structural formulas for given compound is shown in Figure 4. The parent chain ispentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- What is the difference in bonding and in the general molecular formula between an alkene and an alkane with the same number of carbon atoms?arrow_forwardUse the generic formula for alkanes (CnH2n+2) to derive molecular and condensed structural formulas for: a. Propane, 3 carbon atoms b. Octane, 8 carbon atoms c. Butane, 4 carbon atomsarrow_forwardIs the general formula of a cycloalkanes the same as the general formula of an alkane, CnH2n+2? Draw any structural diagram to illustrate your answer.arrow_forward
- Write the molecular formula of each alkane.arrow_forwardWrite the condensed formula (NOT LINE) for the following reactants. a. 6-bromo-2-heptene b. 2-ethyl-1-pentene c. 1-butene d. 3-methyl-2-heptene e. 2-octene f. 4-methyl-1-pentenearrow_forwardDraw the structures of the following hydrocarbons. Use either full structural diagrams or the combination method and don't draw Skeletal or line diagrams.arrow_forward
- Which of the following is a CORRECT name according to the IUPAC rules? a. 2-ethyl-2-methylpentane b. 3,4-dimethylpentane c. 3-ethyl-2-methylpentane d. 2-methylcyclohexanearrow_forwardDraw the carbon skeletal structure for the following organic compounds and identify the main functional group or family. A. 1-methyl 1-cyclopentanol B. 3,4-diethyl nonanal C. 2,3,4,4-tetrabromo-1-octene D. 3-methyl pentanoic acid E. ethyl dimethylaminearrow_forwardA student was given the structural formulas of several compounds and was asked to give them systematic names. How many did she name correctly? Correct those that are misnamed. a. 4-ethyl-2-pentyne b. 1-bromo-4-heptyne c. 2-methyl-3-hexyne d. 3-pentynearrow_forward
- What is the IUPAC name of this compound ? A. 3-ethyl-2,7,8-trimethylnonane B. 2,6-diisopropyloctane C. 6-ethyl-2-isopropyl-7-methyloctane D. 7-ethyl-2,3,8-trimethylnonane E. 7-isopropyl-2,3-dimethylnonanearrow_forwardA student was given the structural formulas of several compounds and was asked to give them systematic names. How many did she name correctly? Correct those that are misnamed. a. 4-ethyl-2-pentyne b. 2-methyl-3-hexyne c. 4-chloro-2-pentyne d. 2,3-dimethyl-5-octyne e. 4-heptynearrow_forwardDraw the structure (Optional Condensed or Line) for the following Organic compounds. By using IUPAC system. A. 2,4-di ethoxy hexane B. 3-bromo-2-floro-3-methy hexanol C. 3,4-dimethyl pentan-2-one D. Fluoro 2-bromo-3-chloro pentanoatearrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





