
Concept explainers
Intermolecular Forces
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these elements reacts with the others.
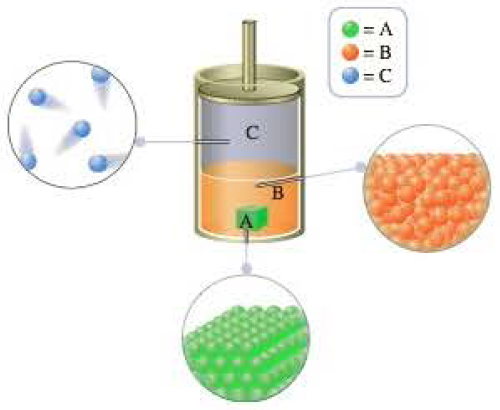
- a What is the state (solid, liquid, or gas) of each of the elements represented in the container?
- b Rank the elements in the container from greatest to least, in terms of intermolecular interactions. Explain your answer.
- c What type(s) of intermolecular attractions are present in each of these elements?
- d Explain which element has the greatest
atomic mass . - e One of the elements in the container has a normal boiling point of 2 K. Which element would that be (A, B, or C)? How do you know?
- f One of the elements has a melting point of 50 K. Which element would that be (A, B, or C)? Why?
- g The remaining element (the one you have yet to choose) has a normal boiling point of 25 K. Identify the element. Could this element have a freezing point of 7 K? Explain.
- h If you started heating the sample to 20 K, explain what you would observe with regard to the container and its contents during the heating.
- i Describe the container and its contents at 20 K. Describe (include a drawing) how the container and its contents look at 20 K.
- j Now you increase the temperature of the container to 30 K. Describe (include a drawing) how the container and its contents look at 30 K. Be sure to note any changes in going from 20 K to 30 K.
- k Finally, you heat the container to 60 K. Describe (include a drawing) how the container and its contents look at this temperature. Be sure to note any changes in going from 30K to 60k
(a)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
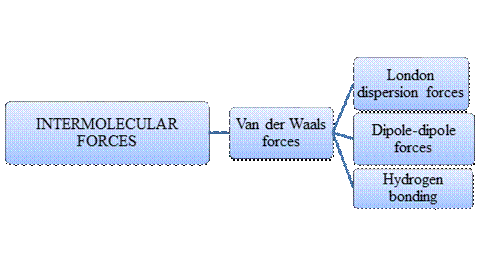
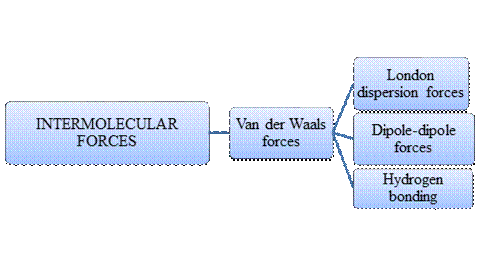
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: the state of each of the elements in the vessel
In a vessel, A is in solid state, B is in liquid state and C is in gaseous state.
Because of solids have definite shape and volume, liquids are definite volume and indefinite shape and gases have both indefinite volume and shape.
(b)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
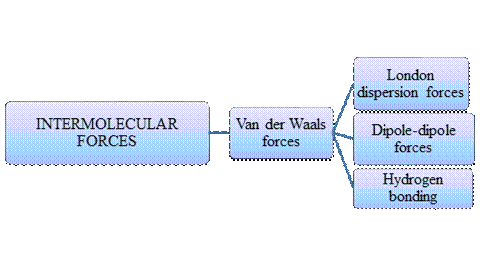
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To rank: the elements from highest to lowest intermolecular attraction
Solids have highest intermolecular attractions than liquids which has greater intermolecular attraction than gases.
Thus, the increasing order of intermolecular attractions is,
(c)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
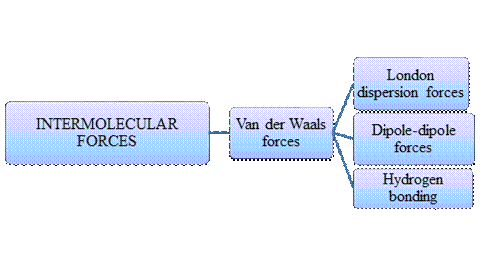
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: intermolecular attractions in each of these elements
Each of the substances are found to be monoatomic non-metal. So London forces only present.
(d)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:
Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: the element has highest atomic mass
The highest atomic mass will be for highly stronger intermolecular forces. Hence, which is found to be A (solid).
(e)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the element has normal boiling point of 2K
The element with normal boiling point of 2K is found to be gas (C). Because of gas is substance whose boiling point is lower than ambient temperature.
(f)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the element has melting point of 50K
The substance with melting point of 50K is found to be A (solid). Because of solids must have melting point be higher than ambient temperature.
(g)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To identify: the remaining element has normal boiling point of 25K
The substance with normal boiling point of 25K is found to be B. Because it cannot freeze at 7K or it should be in solid state. The demand for a substance to be a liquid, the freezing point should be lower than ambient temperature.
(h)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To explain: what happen when sample is heated to 20K regards to vessel
As we start to heat the vessel to 20K, the gas should expand and the piston would move awake.
(i)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: the container and its content at
At
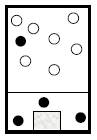
Figure 1
(j)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: how container and its content at 30K and ant changes from 20K to 30K
The temperature is now higher than the boiling point of B which is 25K; therefore both B and C are now gaseous phase. A persists in solid state.
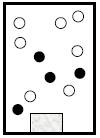
Figure 2
(k)
Interpretation:
Given pictures represents an atom of hypothetical, non-metallic and monoatomic elements A, B and C in a vessel at
Concept introduction:

Intermolecular forces are Van der Waals forces. They are weak and have two types viz., London dispersion forces, dipole-dipole forces and hydrogen bonding. Hydrogen bonding is relatively the strongest one.
- Intermolecular forces are the forces acting between molecules whereas Intramolecular forces are the forces that operate within a molecule.
- Hydrogen bonding is a special type of Dipole-dipole forces but stronger than the former.
- London dispersion forces exist in non-polar covalent compounds whereas dipole-dipole forces exist in polar covalent compounds but both are weak.
- Larger the molecular size, stronger the London dispersion force.
- Arrangement of major types of intermolecular forces in increasing order of strength:
Explanation of Solution
To describe: how container and its content at 60K and ant changes from 30K to 60K
When the temperature of the container attains 60K, the component A has now melted and changed into liquid phase. The gases B and C are continue to enlarge according to Charles’s law.
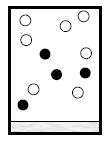
Figure 3
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: General Chemistry, Loose-leaf Version, 11th + OWLv2, 4 terms (24 months) Printed Access Card
- Substance A is composed of molecules that have stronger intermolecular forces than the molecules that compose substance B. Which substance has a lower boiling point? a. substance A b. substance B c. cannot be determined without more information.arrow_forwardRefer to Figure 11.12 to answer these questions: (a) You heat some water to 60 C in a lightweight plastic bottle and seal the top very tightly so gas cannot enter or leave the carton. What happens when the water cools? (b) If you put a few drops of liquid diethyl ether on your hand, does it evaporate completely or remain a liquid? Figure 11.12 Vapor pressure curves for diethyl ether [(C2H3)2O], ethanol (C2H5OH), and water. Each curve represents conditions of T and P of which the two phases, liquid and vapor, are in equilibrium. These compounds exist as liquids for temperatures and pressures to the left of the curve and as gases under conditions to the right of the curve. (See Appendix G for vapor pressures for water of various temperatures.)arrow_forwardThe enthalpy of vaporization of water is larger than its enthalpy of fusion. Explain why.arrow_forward
- The amount of heat required to melt 2 lbs of ice is twice the amount of heat required to melt 1 lb of ice. Is this observation a macroscopic or microscopic description of chemical behavior? Explain your answer.arrow_forwardDefine the joule in terms of SI base units.arrow_forwardCake mixes and other packaged foods that require cooking often contain special directions for use at high elevations. Typically these directions indicate that the food should be cooked longer above 5000ft. Explain why it takes longer to cook something at higher elevations.arrow_forward
- The physical properties of the substances are influenced by intermolecular forces of attraction. Which substance(s) are predominantly influenced by the intermolecular association of the molecules via hydrogen bonding?arrow_forwardThere are five pure substances. The molecular component is shown per substance. Based on this information, answer items 56 to 65. Substance Molecular Component Substance Molecular Component O Substance A Substance D Substance B Substance E Substance C OH 56. The physical properties of the substances are influenced by intermolecular forces of attraction. Which substance(s) are predominantly influenced by the intermolecular association of the molecules via hydrogen bonding? A. Substance A only B. Substance A and C C. Substance A, B, and C D. Substance A, B, C, and D 57. Which substance is expected to turn blue litmus paper into red color? A. Substance A B. Substance B C. Substance C D. Substance D OH Harrow_forwardPlease don't provide handwriting solutionarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





