
Interpretation:
Lewis structure for
Explanation of Solution
Given compounds are
Lewis Structures:
Lewis structure for
The total number of valence electron in carbon is four and that of fluorine is seven. Therefore, the total number of valence electrons in
Skeletal structure for
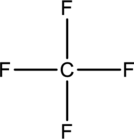
Eight electrons are utilized for the skeletal structure of
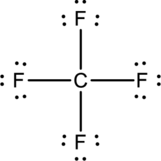
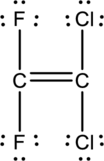
Lewis structure for
The total number of valence electron in carbon is four and that of fluorine, chlorine is seven. Therefore, the total number of valence electrons in
Skeletal structure for
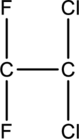
Ten electrons are utilized for the skeletal structure of
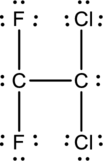
A double bond is made between the two carbon atoms. Therefore, the Lewis structure of
Hybridization:
Hybridization of the carbon atom can be determined by the steric number. Steric number is the total number of lone pair of electrons present on the central atom and the number of atoms that is bonded to the central atom.
Hybridization of carbon atom in
In
Hybridization of carbon atoms in
In
Polar and nonpolar:
The molecule
The molecule
Intermolecular forces:
The molecules
Boiling point:
Strength of the intermolecular forces that is present in
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry: Principles and Practice
- The compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula. Which is expected to have the higher surface tension? Why?arrow_forward8.41 What is the specific feature of N, O, and F that causes them to play a role in hydrogen bonding?arrow_forwardConsider two different organic compounds, each with the formula C2H6O. One of these compounds is a liquid at room conditions and the other is a gas. Write Lewis structures consistent with this observation, and explain your answer. (Hint: The oxygen atom in both structures satisfies the octet rule with two bonds and two lone pairs.)arrow_forward
- 8.39 Under what circumstances are ion-dipole forces important?arrow_forwardThe normal boiling point of SO2 is 263.1 K and that of NH3 is 239.7 K. At −40 °C, would you predict that ammonia has a vapor pressure greater than, less than, or equal to that of sulfur dioxide? Explain.arrow_forwardConsider the iodine monochloride molecule, ICI. Because chlorine is more electronegative than iodine, this molecule is a dipole. How would you expect iodine monochloride molecules in the gaseous state to orient themselves with respect to each other as the sample is cooled and the molecules begin to aggregate? Sketch the orientation you would expect.arrow_forward
- Explain why liquids assume the shape of any container into which they are poured, whereas solids are rigid and retain their shape.arrow_forward8.43 Identify the kinds of intermolecular forces (London dispersion, dipoledipole, or hydrogen bonding) that are the most important in each of the following substances. (a) methane (CH4) , (b) methanol (CH4OH) , (c) chloroform (CHCl3) , (d) benzene (C6H6) , (e) ammonia (NH3) , (f) sulfur dioxide (SO2)arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





