
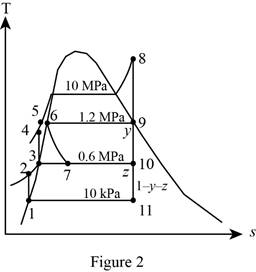
Consider an ideal steam regenerative Rankine cycle with two feedwater heaters, one closed and one open. Steam enters the turbine at 10 MPa and 600°C and exhausts to the condenser at 10 kPa. Steam is extracted from the turbine at 1.2 MPa for the closed feedwater heater and at 0.6 MPa for the open one. The feedwater is heated to the condensation temperature of the extracted steam in the closed feedwater heater. The extracted steam leaves the closed feedwater heater as a saturated liquid, which is subsequently throttled to the open feedwater heater. Show the cycle on a T-s diagram with respect to saturation lines, and determine (a) the mass flow rate of steam through the boiler for a net power output of 400 MW and (b) the thermal efficiency of the cycle.
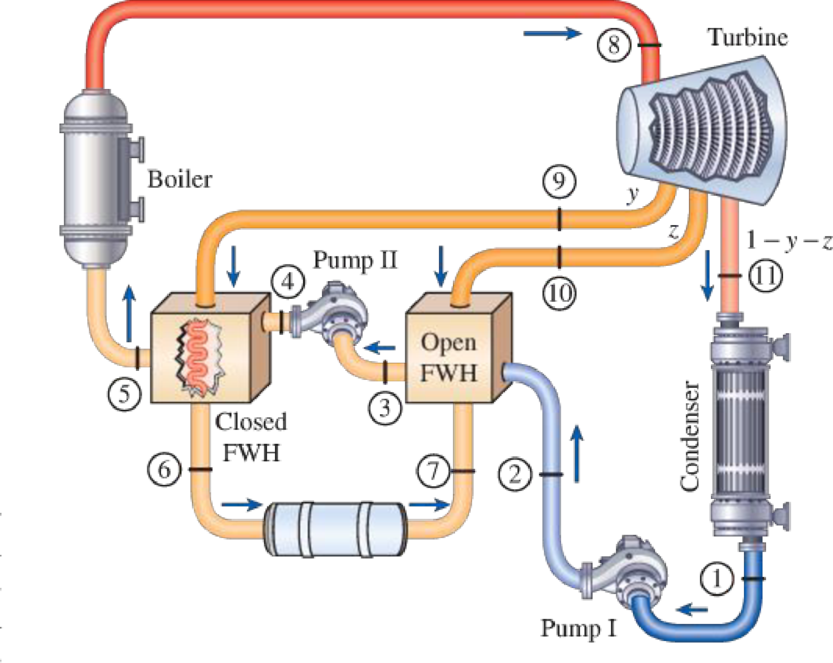
FIGURE P10–53
(a)
The mass flow rate of the steam through the boiler for a net power output of
Answer to Problem 53P
The mass flow rate of the steam through the boiler for a net power output of
Explanation of Solution
Draw the schematic diagram of the given ideal regenerative Rankine cycle as shown in
Figure 1.
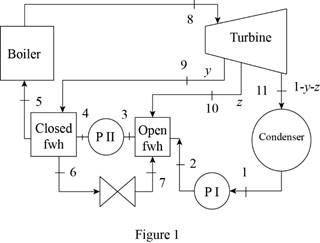
Draw the
Figure 2.
Here, water (steam) is the working fluid of the regenerative Rankine cycle. The cycle involves three pumps.
Write the formula for work done by the pump during process 1-2.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 3-4.
Here, the specific volume is
Write the formula for enthalpy
At state 11:
The steam expanded to the pressure of
The quality of water at state 11 is expressed as follows.
The enthalpy at state 11 is expressed as follows.
Here, the enthalpy is
Refer Figure 1 and 2.
Write the formula for heat in
Here, the mass fraction steam extracted from the turbine to the feed water entering the boiler via closed feed water heater
Write the general equation of energy balance equation.
Here, the rate of net energy inlet is
At steady state the rate of change of net energy of the system
Refer Equation (IX).
Consider the closed feed water heater alone.
Here,
Write the energy balance equation for closed feed water heater.
Rewrite the Equation (X) in terms of mass fraction
Refer Equation (IX).
Consider the open feed water heater alone.
Here,
Write the energy balance equation for open feed water heater.
Rewrite the Equation (XII) in terms of mass fraction
Write the formula for net work output of the cycle.
Write the formula for mass flow rate of the cycle.
At state 1: (Pump I inlet)
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 3: (Pump II inlet)
The water exits the open feed water heater-I as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
At state 6: (boiler inlet or closed feed water exit)
The feed water is heated to the condensation temperature
Refer Table A-5, “Saturated water-Pressure table”.
The temperature
The extracted steam exits the closed feed water heater as a saturated liquid at the pressure of
The enthalpy
At State 5:
The extracted steam exits the closed feed water heater as a saturated liquid at the temperature of
Refer Table A-4, “Saturated water-Temperature table”.
The enthalpy
At state 7:
The steam at state 6 is throttled to state 7. During throttling the enthalpy kept constant.
At state 8:
The steam enters the turbine as superheated vapor.
Refer Table A-6, “Superheated water”.
The enthalpy
From Figure 2,
At state 9:
The steam is extracted at the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state 10:
The steam is extracted at the pressure of
Refer Table A-6, “Superheated water”.
The enthalpy
At state 11:
The steam enters the condenser at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
Obtain the following properties corresponding to the pressure of
Conclusion:
Substitute
Substitute
Substitute
Equation (III).
Substitute
From Figure 1,
Substitute
Substitute
Equation (VI).
Consider the open feed water heater alone.
Substitute
Consider the closed feed water heater alone.
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the mass flow rate of the steam through the boiler for a net power output of
(b)
The thermal efficiency of the cycle.
Answer to Problem 53P
The thermal efficiency of the cycle is
Explanation of Solution
Write the formula for thermal efficiency of the cycle
Conclusion:
Substitute
Thus, the thermal efficiency of the cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- - | العنوان In non-continuous dieless drawing process for copper tube as shown in Fig. (1), take the following data: Do-20mm, to=3mm, D=12mm, ti/to=0.6 and v.-15mm/s. Calculate: (1) area reduction RA, (2) drawing velocity v. Knowing that: ti: final thickness V. Fig. (1) ofthrearrow_forwardA direct extrusion operation produces the cross section shown in Fig. (2) from an aluminum billet whose diameter 160 mm and length - 700 mm. Determine the length of the extruded section at the end of the operation if the die angle -14° 60 X Fig. (2) Note: all dimensions in mm.arrow_forwardFor hot rolling processes, show that the average strain rate can be given as: = (1+5)√RdIn(+1)arrow_forward
- : +0 usão العنوان on to A vertical true centrifugal casting process is used to produce bushings that are 250 mm long and 200 mm in outside diameter. If the rotational speed during solidification is 500 rev/min, determine the inside radii at the top and bottom of the bushing if R-2R. Take: -9.81 mis ۲/۱ ostrararrow_forward: +0 العنوان use only In conventional drawing of a stainless steel wire, the original diameter D.-3mm, the area reduction at each die stand r-40%, and the proposed final diameter D.-0.5mm, how many die stands are required to complete this process. онarrow_forwardIn non-continuous dieless drawing process for copper tube as shown in Fig. (1), take the following data: Do-20mm, to=3mm, D=12mm, ti/to=0.6 and vo-15mm/s. Calculate: (1) area reduction RA, (2) drawing velocity v. Knowing that: t₁: final thickness D₁ V. Fig. (1) Darrow_forward
- A vertical true centrifugal casting process is used to produce bushings that are 250 mm long and 200 mm in outside diameter. If the rotational speed during solidification is 500 rev/min, determine the inside radii at the top and bottom of the bushing if R-2Rb. Take: 8-9.81 m/sarrow_forwardIn conventional drawing of a stainless steel wire, the original diameter D.-3mm, the area reduction at each die stand r-40%, and the proposed final diameter D₁-0.5mm, how many die stands are required to complete this process.arrow_forwardA vertical true centrifugal casting process is used to produce bushings that are 250 mm long and 200 mm in outside diameter. If the rotational speed during solidification is 500 rev/min, determine the inside radii at the top and bottom of the bushing if R-2Rb. Take: 8-9.81 m/sarrow_forward
- In non-continuous dieless drawing process for copper tube as shown in Fig. (1), take the following data: Do-20mm, to=3mm, D=12mm, ti/to=0.6 and vo-15mm/s. Calculate: (1) area reduction RA, (2) drawing velocity v. Knowing that: t₁: final thickness D₁ V. Fig. (1) Darrow_forward-6- 8 من 8 Mechanical vibration HW-prob-1 lecture 8 By: Lecturer Mohammed O. attea The 8-lb body is released from rest a distance xo to the right of the equilibrium position. Determine the displacement x as a function of time t, where t = 0 is the time of release. c=2.5 lb-sec/ft wwwww k-3 lb/in. 8 lb Prob. -2 Find the value of (c) if the system is critically damping. Prob-3 Find Meq and Ceq at point B, Drive eq. of motion for the system below. Ш H -7~ + 目 T T & T тт +arrow_forwardQ For the following plan of building foundation, Determine immediate settlement at points (A) and (B) knowing that: E,-25MPa, u=0.3, Depth of foundation (D) =1m, Depth of layer below base level of foundation (H)=10m. 3m 2m 100kPa A 2m 150kPa 5m 200kPa Barrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





