
Prescott's Microbiology
11th Edition
ISBN: 9781260211887
Author: WILLEY, Sandman, Wood
Publisher: McGraw Hill
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10.4, Problem 1MI
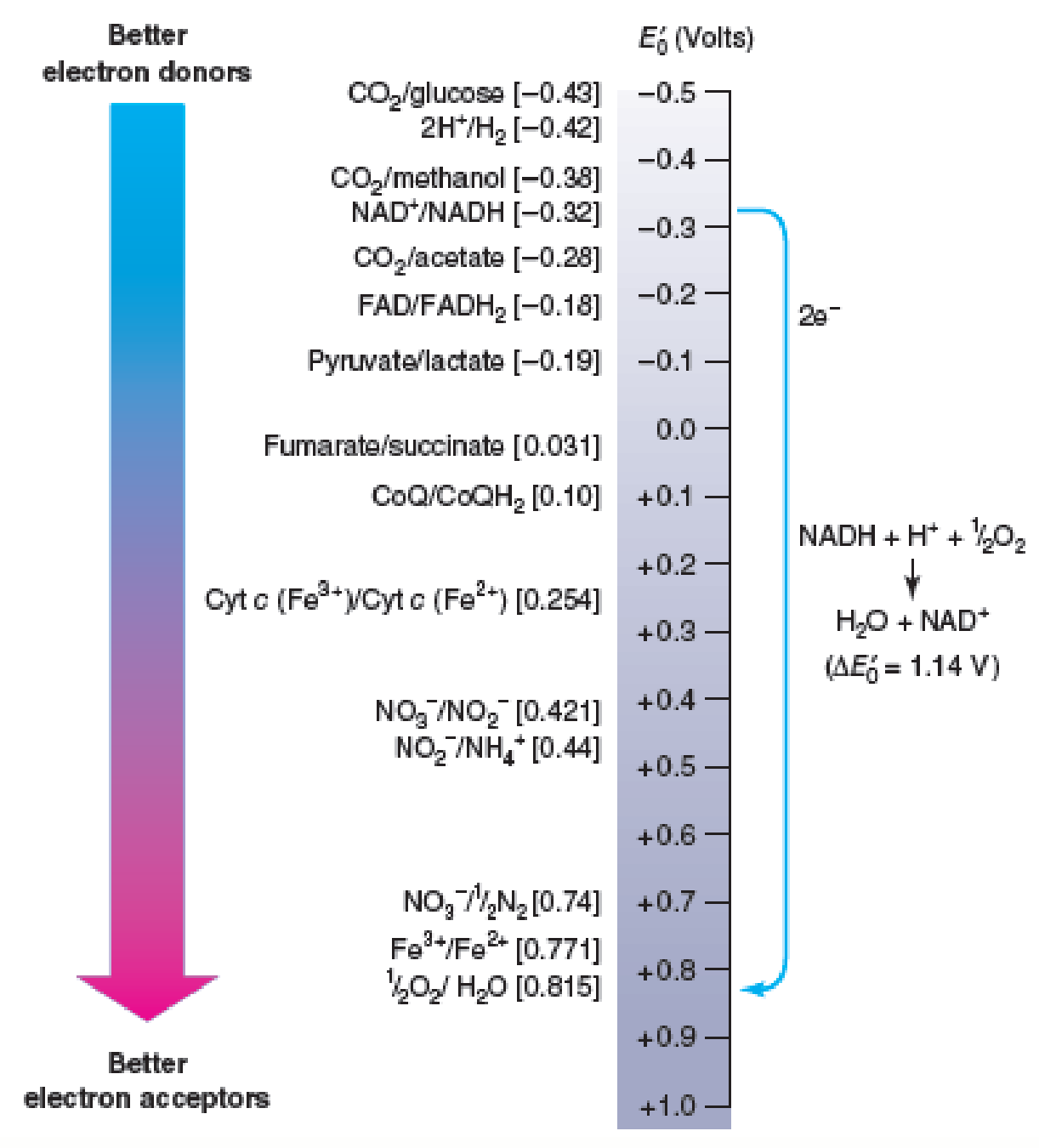
Figure 10.6 Electron Movement and Reduction Potentials. Electrons spontaneously move from donors higher on the tower (more negative potentials) to acceptors lower on the tower (more positive potentials). That is, the donor is always higher on the tower than the acceptor. For example, NADH will donate electrons to oxygen and form water in the process. Some typical conjugate redox pairs are shown on the left, and their reduction potentials are given in brackets.
Refer to figure 10.6 and determine the E′0 for NAD+/NADH and coenzyme Q/CoQH2. Suggest a plausible E′0 value for FMN.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In full details. Explain the significance of redox potentials formed by redox pairs in the electron transport chain.
I don't understand it. Can u help me? Can u help me to explain this to me, please
Fo-F1 ATPase. The energy for ATP synthesis from ADP and Pi is provided by the downhill transport of protons through the rotary FoF1 ATP synthase (lecture 22). The enzyme has 3 a-b and 12 ‘c’ subunits. The mitochondrion maintains Df=180 mV (negative inside), pHin = 8, pHout=7, [Pi] = 3 mM and ADP is present as well.
How much energy is available (from the proton electrochemical gradient) for ATP synthesis under these conditions (in kJ/mol)?
What [ATP]/[ADP] ratio will be established at steady-state under these conditions?
What would be the [ATP]/[ADP] ratio if the enzyme had only 9 ‘c’ subunits? Remember that full revolution of the crank (gamma subunit) produces 3 ATP.
Chapter 10 Solutions
Prescott's Microbiology
Ch. 10.1 - Figure 10.2 The Relationship of G to the...Ch. 10.1 - Prob. 1CCCh. 10.1 - Prob. 2CCCh. 10.1 - Prob. 3CCCh. 10.1 - Prob. 4CCCh. 10.2 - Why is ATP called a high-energy molecule? How is...Ch. 10.2 - Describe the energy cycle and ATPs role in it....Ch. 10.3 - Prob. 1MICh. 10.3 - Prob. 2MICh. 10.4 - Figure 10.6 Electron Movement and Reduction...
Ch. 10.4 - How is the direction of electron flow between...Ch. 10.4 - When electrons flow from the NAD+/NADH conjugate...Ch. 10.4 - Which among the following would be the best...Ch. 10.4 - In general terms, how is G related to E0? What is...Ch. 10.4 - Name and briefly describe the major electron...Ch. 10.6 - Will an enzyme with a relatively high Km have a...Ch. 10.6 - Prob. 2MICh. 10.6 - Prob. 1CCCh. 10.6 - Prob. 2CCCh. 10.6 - How does enzyme activity change with substrate...Ch. 10.6 - What special properties might an enzyme isolated...Ch. 10.6 - What are competitive and noncompetitive...Ch. 10.6 - How are enzymes and ribozymes similar? How do they...Ch. 10.7 - Figure 10.19 Allosteric Regulation. The structure...Ch. 10.7 - Prob. 2MICh. 10.7 - Define the terms metabolic channeling and...Ch. 10.7 - Define allosteric enzyme and allosteric effector.Ch. 10.7 - Prob. 3CCCh. 10.7 - Prob. 4CCCh. 10.7 - Prob. 5CCCh. 10 - Prob. 1RCCh. 10 - Prob. 2RCCh. 10 - Prob. 3RCCh. 10 - Examine the structures of macromolecules in...Ch. 10 - Examine the branched pathway shown here for the...Ch. 10 - Prob. 3AL
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- I don't understand it. Can u help me? Can u help me to explain this to me, pleasearrow_forwardA4. what conformational state is stabilized by y in atp synthase? why might achieving this state require energy input from the pmf?arrow_forwardFo-F1 ATPase. The energy for ATP synthesis from ADP and Pi is provided by the downhill transport of protons through the rotary FoF1 ATP synthase . The enzyme has 3 alpha-beta and 12 ‘c’ subunits. The mitochondrion maintains change in membrane potential=180 mV (negative inside), pHin = 8, pHout=7, [Pi] = 3 mM and ADP is present as well. . What [ATP]/[ADP] ratio will be established at steady-state under these conditions? What would be the [ATP]/[ADP] ratio if the enzyme had only 9 ‘c’ subunits? full revolution of the crank (gamma subunit) produces 3 ATP.arrow_forward
- Identification.arrow_forwardFigure 1 shows the catalytic triad of a-chymotrypsin. Identify A, B and C. Describe the subsequent steps of stage 1 and stage 2 of a- chymotrypsin mechanism. Illustrate with diagrams. A B CH2 H-N N: H-O Figure 1arrow_forwardGive answer all questions with explanation pleasearrow_forward
- Identification.arrow_forwardThe molar absorption coefficient of cytochrome P450. an enzyme involved in the breakdown of harmful substances in the liver and small intestine. at 522 nm is 291 dm3 mol-1 cm-1. When light of that wavelength passes through a cell of length 6.5 mm containing a solution of the solute. 39.8 percent of the light was absorbed. What is the molar concentrat ion of the solute?arrow_forwardActivity of the light reactions (ETC) can be assayed using an artificial, reducible compound (e- acceptor) added to take the place of the terminal electron acceptor. The artificial e- acceptor used is called DCPIP, a blue dye that turns colorless when it accepts electrons. The reaction is: H2O + DCPIPoxidized (blue) --> O2 + DCPIPreduced (colorless) What molecule from the light reactions of photosynthesis does DCPIP compete with? NADPH H2O ATP ADP NADP+arrow_forward
- a What would be the appropriate name for an enzyme that catalyzes each of the following reactions: b H3C iOH OH NH₂ alanine ligase alanine oxidoreductase alanine isomerase alanine transferase OH H3C CH3 H3C NO₂ propanone transferase propan-2-ol hydrolase propan-2-ol oxidoreductase propan-2-ol isomerase OH H3C CH3arrow_forwardOd. Vitamin B2 s page Hexokinase catalyzes phosphorylation of glucose to clucose-6-phosphate, where ATP is used as a donor of phosphate group, this an example of: Select one: NAVIGATION a. Oxidoreductase b. Ligase c. Lyase d. Transferase Next pagearrow_forwardGive only typing answer with explanation and conclusionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Introduction to the NIOSH Manual of Analytical Methods Fifth edition; Author: Centers for Disease Control and Prevention (CDC);https://www.youtube.com/watch?v=B5rUrKLMoas;License: Standard Youtube License