
Inquiry into Physics
8th Edition
ISBN: 9781337515863
Author: Ostdiek
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 13P
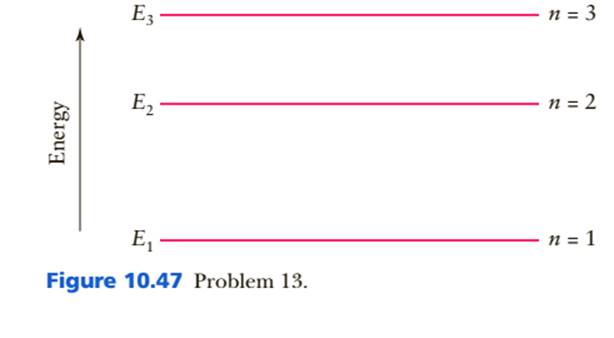
. Figure 10.47 is the energy-level diagram for a particularly simple, fictitious element, Vernium (Vn). Indicate by the use of arrows all allowed transitions leading to the emission of photons from this atom and order the frequencies of these photons from highest (largest) to lowest (smallest).
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Suppose that you have 1 mol of hydrogen atoms in the ground state. If the atoms are irradiated with light, what is the minimum number of photons required to excite all of them? [The answer is a number. Use a decimal point. Round your answer to three decimal places, for example 2.000E-10 or 1.140.] *
Photons from the Balmer series of hydrogen transitions are sent through a double slit.
What must be the distance between the slits such that the lowest energy Balmer transition has a first-order maximum (maximum adjacent to the central maximum) at an angle of
2.00°?
Express your answer in um.
Type your answer...
At what angle, in degrees, would the first maximum be for the fourth-lowest energy Balmer transition photon, when sent through the slits above?
Type your answer...
DOD
Submit
Some of the most powerful lasers are based on the energy levels of neodymium in solids, such as glass, as shown in Figure below.
(a) What average wavelength light can pump the neodymium into the levels above its metastable state?
.(b) Verify that the 1.17 eV transition produces 1.06 micrometre radiation.
Chapter 10 Solutions
Inquiry into Physics
Ch. 10 - Prob. 1SACh. 10 - Prob. 1OACh. 10 - Prob. 1PIPCh. 10 - Prob. 1MIOCh. 10 - Prob. 2MIOCh. 10 - Prob. 1QCh. 10 - Prob. 2QCh. 10 - Prob. 3QCh. 10 - Prob. 4QCh. 10 - Prob. 5Q
Ch. 10 - Prob. 6QCh. 10 - Prob. 7QCh. 10 - Prob. 8QCh. 10 - Prob. 9QCh. 10 - Prob. 10QCh. 10 - Prob. 11QCh. 10 - (Indicates a review question, which means it...Ch. 10 - Prob. 13QCh. 10 - Prob. 14QCh. 10 - (Indicates a review question, which means it...Ch. 10 - Prob. 16QCh. 10 - Prob. 17QCh. 10 - Prob. 18QCh. 10 - Prob. 19QCh. 10 - Prob. 20QCh. 10 - Prob. 21QCh. 10 - Prob. 22QCh. 10 - Prob. 23QCh. 10 - Prob. 24QCh. 10 - Prob. 25QCh. 10 - Prob. 26QCh. 10 - Prob. 27QCh. 10 - Prob. 28QCh. 10 - Prob. 29QCh. 10 - Prob. 30QCh. 10 - Prob. 31QCh. 10 - Prob. 32QCh. 10 - Prob. 33QCh. 10 - Prob. 34QCh. 10 - Prob. 35QCh. 10 - Prob. 36QCh. 10 - Prob. 37QCh. 10 - Prob. 38QCh. 10 - Prob. 39QCh. 10 - Prob. 40QCh. 10 - Prob. 41QCh. 10 - Prob. 42QCh. 10 - Prob. 1PCh. 10 - Prob. 2PCh. 10 - Prob. 3PCh. 10 - Prob. 4PCh. 10 - Prob. 5PCh. 10 - Prob. 6PCh. 10 - Prob. 7PCh. 10 - Prob. 8PCh. 10 - Prob. 9PCh. 10 - Prob. 10PCh. 10 - Prob. 11PCh. 10 - Prob. 12PCh. 10 - . Figure 10.47 is the energy-level diagram for a...Ch. 10 - Prob. 14PCh. 10 - Prob. 15PCh. 10 - Prob. 16PCh. 10 - Prob. 17PCh. 10 - Prob. 18PCh. 10 - Prob. 19PCh. 10 - Prob. 20PCh. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 1CCh. 10 - Prob. 2CCh. 10 - The rate at which solar wind particles enter the...Ch. 10 - Prob. 4CCh. 10 - Prob. 5CCh. 10 - Prob. 6C
Additional Science Textbook Solutions
Find more solutions based on key concepts
The formula for the sum Sn of the geometric series Sn=a+ar+.....arn−1 .
Mathematical Methods in the Physical Sciences
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
GO You testify as an expert witness in a case involving an accident in which car A slid into the rear of car B,...
Fundamentals of Physics Extended
An electric motor has an effective resistance of 32.0 and an inductive reactance of 45.0 when working under l...
Fundamentals Of Physics - Volume 1 Only
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
The Physics of Everyday Phenomena
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 4, 3. Excited Ground state 1(ground state) state 4. 4.85E-19 J 4.42E-19 J 3.98E-19 J 3. 1. 3.03E-19 J 2. 1. 1 (ground state) Energy Energy paquosqe emitted 2) [30] Energy Levels Above is a schematic of a Hydrogen atom with its first 5 energy levels. On the right is the energy emitted from the transitions (lines pointing down on the diagram). Using the knowledge that energy and wavelength are hc, connected ( E =) you will figure out the wavelength for each of these %3D transitions. %3D E. h = Planck constant = 6.63E-34 J*s c = speed of light = 3E8 m/s 2 = wavelength in meters E = energy in Joules (J) %3D hc %3D E will be in meters! Divide by 10-9 for nm If you need help converting this to a color easier, try this website once you get the wavelength in nm: https://academo.org/demos/wavelength-to- colour-relationship/arrow_forwardIdentify the initial and final states if an electron in hydrogen emits a photon with a wavelength of 656 nm. Enter your answers numerically separated by a comma.arrow_forwardSolve the following, if a television signal (video and audio) has a bandwidth of 4.5MHz, this signal is sampled, quantized, and binary coded to obtain a PCM signal. I. If the signal is to be sampled at a rate 30% above the Nyquist rate, then sampling rate?II. If the samples are quantized into 2048 levels, determine the number of binary pulses required to encode each sample.III. Determine the binary pulse rate (bits per second) of the binary-coded signal.IV. Minimum bandwidth required to transmit this signal. V. Find the channel capacity using Nyquist rule.VI. Find the SNR using Shannon rule.arrow_forward
- Consider the atomic spectra for the H-atom: the Lyman series emits UV photons, the Balmer series emits visible photons, the Paschen series emits IR photons, and the Brackett series emits far IR photons. What type of photons would you expect from the next series? Briefly explain.arrow_forwardThe energy difference between the two levels arising from the spin-orbit coupling in a d-orbital is 0.6463 eV. Consider the splitting for one electron. Calculate the spin-orbit coupling constant in cm-1. please show all calculations. What atom could that be? (Hint: calculate the spin-orbit coupling constant in Ry and look for a matching number in the last column that provides experimental values) constant : 1 eV = 1.60217646 × 10-19 J 1Ry = 2.1798741 × 10-18 Jarrow_forward4.8. Solve Schrödinger's equation for the ground state of helium neglecting the potential term between the two electrons. What is the energy of the ground state calculated this way? The measured value is -24.6 eV. What do you think the difference is caused by? Give an explanation in physical terms.arrow_forward
- Physical Chemistry. Calculate the wave function, probability density and probability of finding an electron in the1s orbital of a hydrogen atom at (a) r=0 and (b) r=3/2 ao. Show all steps and units in the calculation.arrow_forward1ls it truc that the Spin of electron means that the electron is aclually Spinning/ rotaling ds axis? on b. can the expectation value of a raising operator be measuned in erpeninent ? an of photons cmitted by a hydro gen why not? [n= integer, f- frequency C E- hf (n+ ) is the energy atom . Is that true ? why or d. Evaluate I(ê) = d. Evalvate I(ê) = (e-alpi+iê?, d'?, P- 3o Positión vector for Some vector (k)arrow_forwardWhen Franck–Hertz experiment was conducted on Sodium vapor , the special sodium line emitted with wavelength of 5896 A , If this line represents the transition from first excitement level to ground level. Explain first and last peak places that show in the experiment. Knowing ( sodium Ionization energy is 5.1 ev) ?arrow_forward
- Choose the correct answer!. 1. The wavelength associtated with a particle in • 2-D box of length I is 2.L L @ © n n 2n Ju • 2. At boundary Condition @√(x) is always continuous except © Vcx = constant. where 9x 16₁ VCX) = 0 = 00 3. The Zero Point energy of the 1-dimensional box occurred with @n=1 n=0 ©n=30 4- In the eigen value equation, the eigen Values is determined by : @Operatory Only Condition ony by boundry by Operatory plus. the boundary conditionsarrow_forwardEx. 1 : An electron revolves around a nucleus with frequency of revolution 1015 Hz. If the radius of orbit is 0.53 A . Calculate the magnetic moment of electron.arrow_forwardIn the PIB model what imposes quantization? Explain. (hint – consider the boundary conditions.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY