
Chemistry
7th Edition
ISBN: 9780321940872
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.23CP
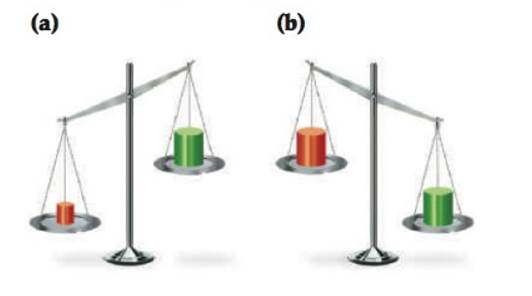
Which block in each of the following drawings of a balance is more dense, red or green? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lorena placed a cup with a stick of butter in it on one side of a balance and a melted stick of butter of the SAME SIZE on the other side of the balance. Choose the picture that correctly shows what the balance would looks like
a Assume that mitochondria are cylinders 8.00 um in length and 0.800 pm in diameter.
What is the volume of a single mitochondrion?
The volume of a cylinder is given by the expression:
V = n x y2 x h
Volume=
m
Direction: Identify each mixture as either homogeneous or
heterogeneous.
1. Orange juice
2. Smoke
3. Paint
4. Soda
5. Steel
Chapter 1 Solutions
Chemistry
Ch. 1 - PRACTICE 1.1 Express the following quantities in...Ch. 1 - APPLY 1.2 Express the following quantities in...Ch. 1 - PRACTICE 1.3 The melting point of table salt is...Ch. 1 - Prob. 1.4ACh. 1 - PRACTICE 1.5 Chloroform, a substance once used as...Ch. 1 - APPLY 1.6 You are beachcombing on summer vacation...Ch. 1 - PRACTICE 1.7 Some radioactive materials emit a...Ch. 1 - Prob. 1.8ACh. 1 - Prob. 1.9PCh. 1 - Prob. 1.10A
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12ACh. 1 - Prob. 1.13PCh. 1 - APPLY 1.14 A sodium chloride solution was prepared...Ch. 1 - PRACTICE 1.15 Gemstones are weighed in carats,...Ch. 1 - PRACTICE 1.15 Gemstones are weighed in carats,...Ch. 1 - Prob. 1.17PCh. 1 - APPLY 1.18 How large, in cubic centimeters, is the...Ch. 1 - Prob. 1.19PCh. 1 - PROBLEM 1.20 Calculate the percentage Of atoms on...Ch. 1 - Prob. 1.21PCh. 1 - Prob. 1.22PCh. 1 - Which block in each of the following drawings of a...Ch. 1 - Prob. 1.24CPCh. 1 - How many milliliters of water does the graduated...Ch. 1 - Assume that you have two graduated cylinders, one...Ch. 1 - The following cylinder contains three liquids that...Ch. 1 - The following statements pertain to the...Ch. 1 - The following statements pertain to the...Ch. 1 - Prob. 1.30SPCh. 1 - Prob. 1.31SPCh. 1 - Prob. 1.32SPCh. 1 - Prob. 1.33SPCh. 1 - Prob. 1.34SPCh. 1 - Prob. 1.35SPCh. 1 - 1.36 What is the difference between mass and...Ch. 1 - Prob. 1.37SPCh. 1 - Prob. 1.38SPCh. 1 - Prob. 1.39SPCh. 1 - Prob. 1.40SPCh. 1 - Prob. 1.41SPCh. 1 - Prob. 1.42SPCh. 1 - Prob. 1.43SPCh. 1 - Prob. 1.44SPCh. 1 - Prob. 1.45SPCh. 1 - Prob. 1.46SPCh. 1 - Prob. 1.47SPCh. 1 - Prob. 1.48SPCh. 1 - Prob. 1.49SPCh. 1 - How many picograms are in 1 mg? In 35 ng?Ch. 1 - Prob. 1.51SPCh. 1 - Prob. 1.52SPCh. 1 - Prob. 1.53SPCh. 1 - How many significant figures are in each of the...Ch. 1 - Prob. 1.55SPCh. 1 - Prob. 1.56SPCh. 1 - Prob. 1.57SPCh. 1 - Prob. 1.58SPCh. 1 - Prob. 1.59SPCh. 1 - Prob. 1.60SPCh. 1 - Prob. 1.61SPCh. 1 - Prob. 1.62SPCh. 1 - Prob. 1.63SPCh. 1 - Prob. 1.64SPCh. 1 - Prob. 1.65SPCh. 1 - Carry Out the following conversions: (a) How many...Ch. 1 - Prob. 1.67SPCh. 1 - Prob. 1.68SPCh. 1 - Prob. 1.69SPCh. 1 - Weights in England are commonly measured in...Ch. 1 - Prob. 1.71SPCh. 1 - Prob. 1.72SPCh. 1 - Prob. 1.73SPCh. 1 - The normal body temperature of a goat is 39.90C...Ch. 1 - Prob. 1.75SPCh. 1 - Prob. 1.76SPCh. 1 - Prob. 1.77SPCh. 1 - Suppose you were dissatisfied with both Celsius...Ch. 1 - Answer parts (a)-(d) of Problem 1.78 assuming that...Ch. 1 - Prob. 1.80SPCh. 1 - Prob. 1.81SPCh. 1 - Prob. 1.82SPCh. 1 - Prob. 1.83SPCh. 1 - Prob. 1.84SPCh. 1 - The density Of silver is 10.5 g/cm3. What is the...Ch. 1 - Prob. 1.86SPCh. 1 - Prob. 1.87SPCh. 1 - Prob. 1.88SPCh. 1 - An experiment is performed to determine if pennies...Ch. 1 - Which has more kinetic energy, a 1400 kg car...Ch. 1 - Prob. 1.91SPCh. 1 - Prob. 1.92SPCh. 1 - Prob. 1.93SPCh. 1 - Prob. 1.94SPCh. 1 - Prob. 1.95SPCh. 1 - Prob. 1.96CPCh. 1 - Lignum vitae is a hard, durable, and extremely...Ch. 1 - Prob. 1.98CPCh. 1 - Prob. 1.99CPCh. 1 - Prob. 1.100CPCh. 1 - Prob. 1.101CPCh. 1 - Answer the following questions: (a) An old rule of...Ch. 1 - A 1.0 ounce piece of chocolate contains 15 mg of...Ch. 1 - Prob. 1.104CPCh. 1 - Prob. 1.105CPCh. 1 - Prob. 1.106CPCh. 1 - Prob. 1.107CPCh. 1 - Prob. 1.108CPCh. 1 - Prob. 1.109CPCh. 1 - Prob. 1.110CPCh. 1 - Prob. 1.111CPCh. 1 - Brass is a copper—zinc alloy. What is the mass in...Ch. 1 - Prob. 1.113CPCh. 1 - The element gallium (Ga) has the second largest...Ch. 1 - Distances over land are measured in statute miles...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.82 Which of the following molecular-scale diagrams best represents a pure compound? Explain your answer.arrow_forwardSeawater is composed of salt, sand, and water. Is seawatera heterogeneous or homogeneous mixture? Explain.arrow_forwardYou receive a mixture of table salt and sand and have to separate the mixture into pure substances. Explain how you would carry out this task. Is your method based on physical or chemical properties? Explain.arrow_forward
- 1) State your interpretation of the meaning of the slope for the first graph you prepared of Volume of Steel Nuts (mL) versus Number of Steel Nuts. Hint: Understanding the meaning of a slope involves several factors: the units which are a ratio of the y axis units over the x axis units, the interpretation of the slope as “number of the y axis units for every one of the x axis units” and the concept that constructing the best line or curve that follows the trend in the data is a sort of averaging of the random error throughout the data.arrow_forwardCalculating the density of a rectangular wooden block (fig 3). In determining the density of a rectangular wooden block, use the information in the table below and the formula to fill in the empty boxes. (Apply the uncertainty rule) Fig 3. Volume of wooden block (cm) = Length (cm) x Width (cm) x Thickness (cm) Mass (g) of wooden block (g) Length of wooden block (cm) Width of wooden block (cm) Thickness of wooden block (cm) Volume of wooden block (cm) Density of wooden block (g/cm) d= m/v 20.5g 5.00cm 5.00cm 1.00 cm 2arrow_forwardClassify these substances. More than one answer may apply in each case. H2 pure substance element element homogeneous mixture heterogeneous mixture compound homogeneous mixture solution solution heterogeneous mixture compound O pure substance H,0 salt water heterogeneous mixture homogeneous mixture O pure substance Oheterogeneous mixture O solution U pure substance homogeneous mixture I element compound O solution element O compound 口□ 口口ㄩarrow_forward
- classify whether it is greater than, less than, or equalarrow_forwardHow can you distinguish an element, from a compound, from a solution, from a heterogeneous mixture? Explainarrow_forwardWater has a density of 1.00g/mL. How many teaspoons of water are represented by 40,500 molecules of water. Use dimensional analysis.arrow_forward
- How many grams F in 650.1 g Br2F6? Put the answer in the box in regular (non-scientific) notation. Use one figure past the decimal.arrow_forward1a) A children's liquid medicine contains 100 mg of the active ingredient in 5 mL. If a child should receive 200 mg of the active ingredient, how many milliliters of the medicine should the child be given? For the purposes of this question, assume that these numbers are exact. 1b)The package states that one teaspoon (tsp) is approximately equal to 5 mL. Calculate the number of teaspoons that the child should receive. Use the given approximation as your conversion factor.arrow_forwardThe mass of magnesium used in this experiment is critical to determining the moles of hydrogen gas generated. The analytical balances used in Chemistry 1A labs are very sensitive to point that touching materials with your fingers before measuring them can affect the measurements. The balances are sensitive enough to detect dirt and oils from your fingers transferred to the magnesium. Would your experimental value of R increase or decrease if the mass of magnesium measured was artificially high due the touching it with your hands? Increase Decreasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY