
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
You have two G’s and two temperatures. Use these to solve for H and S.
TEMP1 - 296.57 K
TEMP2 - 340 K
G1 - 24.65
G2 - 32.82
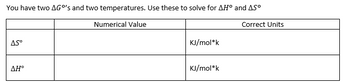
Transcribed Image Text:You have two AGO's and two temperatures. Use these to solve for AH° and AS°
Numerical Value
Correct Units
AS°
AH°
KJ/mol*k
KJ/mol*k
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- When a gas expands, what is the sign of w? Why? When a gas contracts, what is the sign of w? Why? What are the signs of q and w for the process of boiling water?arrow_forward9.47 If 14.8 kJ of heat is given off when 1.6 g of HCl condenses from vapor to liquid, what is Hcond for this substance?arrow_forwardWould the amount of heat absorbed by the dissolution in Example 5.6 appear greater, lesser, or remain the same if the heat capacity of the calorimeter were taken into account? Explain your answer.arrow_forward
- 9.54 The phase change between graphite and diamond is difficult to observe directly. Both substances can be hurned, however. From these equations, calculate H for the conversion of diamond into graphite. C(s,graphite)+O2(g)CO2(g) H= -393.51 kJ C(s,diamond)+O2(g)CO2(g) H= -395.94 kJarrow_forwardPrinciples of Heat Flow Titanium is a metal used in jet engines. Its specific heat is 0.523 J/g C. If S.SS g of titanium absorb 4.78 J, what is the change in temperature?arrow_forwardThe enthalpy of combustion of solid carbon to form carbon dioxide is 393.7 KJ/mol carbon, and the enthalpy of combustion of carbon monoxide to form carbon dioxide is 283.3 KJ/mol CO. Use these data to calculate H for the reaction 2C(s)+O2(g)2CO(g)arrow_forward
- The statement Energycan beneithercreatednor destroyedis sometimes used as an equivalent statement of the first law of thermodynamics. There areinaccuracies to the statement, however. Restate it tomake it less inaccurate.arrow_forwardThe reaction SO3(g)+H2O(l)H2SO4(aq) is the last step in the commercial production of sulfuric acid. The enthalpy change for this reaction is 227 kJ. In designing a sulfuric acid plant, is it necessary to provide for heating or cooling of the reaction mixture? Explain.arrow_forwardThe enthalpy change for the reaction CH4(g)+2O2(g)CO2(g)+2H2O(l) is 891 kJ for the reaction as written. a. What quantity of heat is released for each mole of water formed? b. What quantity of heat is released for each mole of oxygen reacted?arrow_forward
- How is the sign of q, heat, defined? How does it relate to the total energy of the system?arrow_forwardHow much will the temperature of a cup (180 g) of coffee at 95 C be reduced when a 45 g silver spoon (specific heat 0.24 J/g C) at 25 C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water.arrow_forward9.103 One reason why the energy density of a fuel is important is that to move a vehicle one must also move its unburned fuel. Octane is a major component of gasoline. It burns according to the reaction 2C8H18(l)+25O2(g)16CO2(g)+18H2O(g) H = 1.10104 kJ Starting from this thermochemical equation, describe how you would determine the energy density, in kJ/g, for octane. Be sure to indicate what you would need to calculate or look up to complete this problem.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax