
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
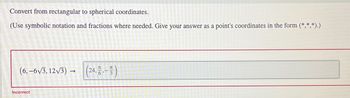
Transcribed Image Text:Convert from rectangular to spherical coordinates.
(Use symbolic notation and fractions where needed. Give your answer as a point's coordinates in the form (*,*,*).)
(6,-6√√3,12√3) →
->
Incorrect
元
6
(24, 17.-113)
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Write the expression for Keq for the following reactions. In each case indicate whether the reaction is homogeneous or heterogeneous.(a) N2(g) + 3 H2(g) 2 NH3(g) Keq = PNH32 / (PN2 PH23) Keq = (PN2) (3 PH2) / 2 PNH3 Keq = PN2 PH23 / PNH32 Keq = 2 PNH3 / ((PN2) (3 PH2)) homogeneous or heterogeneous. (b) 4 HCl(g) + O2(g) 2 H2O(l) + 2 Cl2(g) Keq = PHCl4 PO2 / PCl22 Keq = 2 PCl2 / (4 PHCl PO2) Keq = 4 PHCl PO2 / 2 PCl2 Keq = PCl22 / (PHCl4 PO2)arrow_forward1. The mobile phase in the TLC simulator uses a mixture of the organic compounds: ethyl acetate and hexane. For each compound: circle the polar and nonpolar regions of the molecule, then label each region with the type of attractive force it could use to interact with neighboring molecules. ethyl acetate H :0: Η Η +4 Η H- H Η hexane H- Η Η Η Η Η Η C -C-H H H H H H Harrow_forwardphysical chemistry Roughly, what is the error introduced by the solution of some of the CO2 in the water in the bomb? The solubility of CO2 is about 0.0015 g/mL at 25°C and 1 atm CO2 pressure. The heat of solution of CO2 in a dilute aqueous solution is roughly -19.4 kJ/mol. Assume that the final CO2 pressure is 2 atm and that Henry's Law applies.arrow_forward
- Aa.17.arrow_forward(2) Sally starts eluting her column with petroleum ether in hopes of separating her 50:50 ferrocene: fluorenone mixture. She notices her mobile phase getting low when there is limited separation between her two components. She eagerly adds more solvent to avoid her column from drying out. Sally then realizes she poured from her 1:1 petroleum ether: diethyl ether beaker rather than her petroleum ether beaker. (a) Using the molecular view below, explain how this would impact her product elution from the column. top of column H buret wall AI H H H H H H H H H H bottom of column buret wall (b) How does this error impact her experimental results? (c) Using pictures and words, how could Sally analyze the purity of her product? Fearrow_forward12:27 2) milliliters 13.10 TEMU B. Convert 1.90 gallons to each of the following. Show your set ups. elszovee Report answers in decimal notations with the proper number of significant figure. 1) liters 3) quarts NJD Send a Chat ||| O P 44% اللهarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY