
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
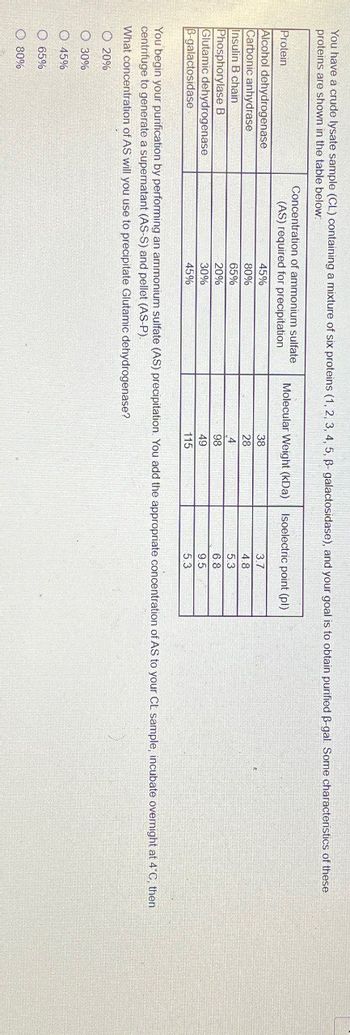
Transcribed Image Text:You have a crude lysate sample (CL) containing a mixture of six proteins (1, 2, 3, 4, 5, ẞ- galactosidase), and your goal is to obtain purified ẞ-gal. Some characteristics of these
proteins are shown in the table below.
Protein
Alcohol dehydrogenase
Carbonic anhydrase
Insulin B chain
Phosphorylase B
Glutamic dehydrogenase
B-galactosidase
45%
Concentration of ammonium sulfate
(AS) required for precipitation
Molecular Weight (kDa)
Isoelectric point (pl)
38
3.7
80%
65%
20%
30%
45%
28
4.8
4
5.3
98
6.8
49
9.5
115
5.3
You begin your purification by performing an ammonium sulfate (AS) precipitation. You add the appropriate concentration of AS to your CL sample, incubate overnight at 4°C, then
centrifuge to generate a supernatant (AS-S) and pellet (AS-P).
What concentration of AS will you use to precipitate Glutamic dehydrogenase?
© 20%
O 30%
45%
65%
80%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- There are eighteen (18) rows of boxes, for around 36-38 boxes total. Please see images attached. This is a pathway trace, with the final box showing "ribose 5 phosphate" as the end product. Any help is appreciated-thank you! glutamate to ribose 5 phosphate No a ketoglutarate dehydrogenase No pyruvate carboxylase No transketolase No transaminase You do get to have if needed: erythrose 4 phosphate asparate dihydroxyacetone phosphate bicarbonate ATP ornithine CO2arrow_forwardHow many ATP are produced from the complete degradation of 1,3-bisphosphoglycerate? the complete degradation of ONE glucose using the malate-aspartate shuttle as your guide.arrow_forwardDefine a ketogenic diet and give an example of a ketogenic meal. Explain why ketone supplements would not be a useful alternative to the ketogenic diet as a cancer therapy. Why might dietary treatments be preferred over conventional treatment options?arrow_forward
- Degradation of normal glycogen results to 97.2% glucose 1-phosphate and 7.2% glucose. Assuming a ratio of glucose 1-phosphate to glucose recorded in a glycogen sample from a patient with a liver disease was 100, The patient’s most likely enzymatic deficiency is _________arrow_forwardPlease explain why the answer is correct and why the other options are incorrectarrow_forwardOutline the mechanism of the conversion of a-ketoglutarate to succinylCoA catalyzed by α-ketoglutarate dehydrogenase complex. Include all products, coenzymes, and reactions in your discussion.arrow_forward
- Indicate what will happen (increase, decrease or no effect) to the activity of the enzyme or rate of the metabolic pathway given the following conditions.arrow_forwardIn class, I mentioned that fructose is metabolized differently in the liver compared to glucose. Refer to the figure shown below to calculate the number ofATPs you would expect from the metabolism of fructose in the liver. Show your work! Fructokinase Fructose Fructose-1-P АТР ADP Aldolase B Dihydroxy- acetone phosphate Glyceraldehyde АТР Triose kinase Triose phosphate isomerase ADP 4 - Glyceraldehyde-3-P Glycolysis Руruvate Acetyl-CoA Fatty acids and triglyceridesarrow_forwardSelect the single best answer. What reaction does the enzyme triose phosphate isomerase catalyze? Conversion of dihydroxyacetone phosphate into glyceraldehyde-3-phosphate. Isomerization of 3-phosphoglycerate into 2-phosphoglycerate. Substrate level phosphorylation involving transfer of a phosphoryl group from 1,3-bisphosphoglycerate into 3-phosphoglycerate. Isomerization of glucose-6-phosphate into fructose-6-phosphate.arrow_forward
- Which of the following would result in an increase in the amount of F-2,6-BP? (select all that apply) Group of answer choices high blood glucose activation of fructose bisphosphatase 2 increased phosphofructokinase 2 activity increased glucagon secretion increased phosphorylation of the bifunctional enzymearrow_forwardName some HMG CoA reductase inhibitors? Please answer at your own words.arrow_forwardThe name of the enzyme that catalyzes the conversion of ATP to CAMP is: CAMP synthase Adenylyl cyclase Phosphodiesterase Pyrophosphate OO 00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON