
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please answer the question correctly is all I want and explain why its thats answer for the final and please answer as quickly as possible, thanks!
You graph the calibration curve of a solution of an unknown metal (we will call it M+) at 400 nm. The graph is shown below. What is the concentration the solution with an absorbance of 0.500 at 400 nm?

Transcribed Image Text:140 mmol/L
1.6 mmol/L
165 mmol/L
330 mmol/L
Impossible to answer with the information given
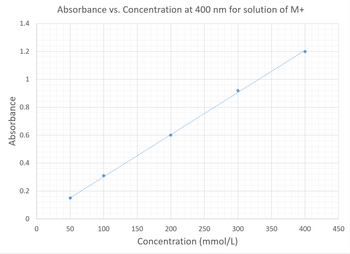
Transcribed Image Text:Absorbance
1.4
1.2
1
0.8
0.6
0.4
0.2
0
0
Absorbance vs. Concentration at 400 nm for solution of M+
50
100
150
200
Concentration (mmol/L)
250
300
350
400
450
Expert Solution
arrow_forward
Step 1
To calculate value of concentration, we can use the graph. For this use the given value of absorbance and draw an straight line to the line of the curve. Then draw an line to the x-axis. This would give value of concentration at given absorbance.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given: You mix together 3 mL of Al3+ (3.00 x 10-5 M) and 3 mL of xylenol orange (5.00 x 10-5 M). The absorbance at room temperature is 0.1 and the absorbance at 65oC is 0.35. a. What is the concentration of AlQ- at 65 degrees Celcius?arrow_forwardSample #1 has an absorbance of 0.300. Sample #2 is the same substance and has an absorbance of 0.600 using the same equipment. What is the relative concentration of the second sample? Select one: a. The concentration of sample #2 is the square root of that of #1. b. The concentration of sample #2 is that of sample #1 squared. c. The concentration of sample #2 is half that of sample #1. d. The concentration of sample #2 is twice that of sample #1.arrow_forward3. Using the graph provided, find the concentration of a solution that had an absorption of 0.269 when measured at 460 nm in a 1.0 cm cuvette. Absorbance vs. Concentration 0.45 0.4- y = 4.07x - 0.0046 R = 0.99353 0.35- %3D 0.3- 0.25 0.2- 0.15 - 0.1- 0.05 0.000 0.020 0.040 0.060 0.080 0.100 0.120 Concentration (mM) Absorbance at 460 nmarrow_forward
- 2) In a laboratory study, a calibration was performed using NH4C1 to analyze ammonium spectrophotometrically. The results of the study are given below. According to this, when the 5.0 mL sample containing ammonium is completed to 50 mL with pure water, find the amount of NH4 in the sample whose absorbance was found as 0.12 according to the table. (N: 14 g / mol, H: 1 g / mol, Cl: 35.5 g / mol). Concentration (mg/L) Absorbance 0.01 5 0.05 7.5 0.1 10 0.15 12 0.18arrow_forwardA standard curve of absorbance vs. concentration has a linear trendline of y = 0.56x + 0.987. Use this trendline to calculate the concentration of an unknown sample that has an absorbance of 1.245.arrow_forwardCan you help me on questions 2 AND 3arrow_forward
- Hh.130.arrow_forward2. ] You make 150.0 mL of a copper(II) sulfate solution but forget to cover it before leaving for the day. The next class, you measure the volume and find that you only have 132.0 mL remaining. You didn’t record the initial concentration, but you measure the absorbance of the remaining solution at 620 nm and find that it is 0.386. You also construct the following calibration curve for CuSO4: Standard solution Abs620 0.50 M 0.424 0.40 M 0.336 0.30 M 0.247 0.20 M 0.159 (refer to image) a. What is the value for k (in Beer’s Law)? Provide a calculation or graph to support your answer. b. What is the concentration of the (remaining) solution? c. What was the concentration of the original solution from the first class? d. If you removed 7.5 mL from the 132.0 mL solution in the process of measuring the absorbance, how much water should you add to obtain the original concentration that you solved for in part (c)?arrow_forwardCreate a calibration curve for the following 4 M concentrations. (0.16 M, 0.080 M, 0.040 M, and 0.016 M.) The data is in the attached image. Note* only use the absorbance points from the highlighted (highlighted in blue) column. It is the column with an absorbance of 442.6.arrow_forward
- A solution of a dye was analyzed by spectrophotometry, and the following calibration data were collected using a 1 cm cuvette. *graph in attachemnt* Using the calibration data above for the dye solution, what is the dye concentration in a solution with an absorbance (A) = 0.52 if it was measured in a 1 cm cuvette? Group of answer choices 3.0 x 10-6 M 6.0 x 104 M 2.0 x 10-6 M Not enough information is provided.arrow_forwardPlease helparrow_forwardFollow-up questions 1. What is the purpose of the lid on the colorimeter? 2. In part 1, why is it necessary to calibrate absorbance at every wavelength? 3. In part 1, the concentration of the CV+ stock solution was 12.5 uM. What mass of CV+ is found in 1 liter of this solution? Give your answer in milligrams. (The molar mass of crystal violet is 407.979g.) 6. 4. What are the effects of the following changes on molar absorptivity? (a) when the concentration of CV+ changes? (b) during the course of the color-loss reaction? (c) when absorbance is measured at different wavelengths? 5. In part 2, what labware or apparatus would you use to prepare solutions for the standard curve? In part 3, what percentage of the NaOH is left at the end of the color-loss reaction, assuming all the crystal violet has reacted? Show your calculation. 6. 7. (a) In part 3, how long is the half-life of the color-loss reaction (show the calculation)? (b) In part 4, is the half-life of the color-loss reaction…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY