
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hh.130.
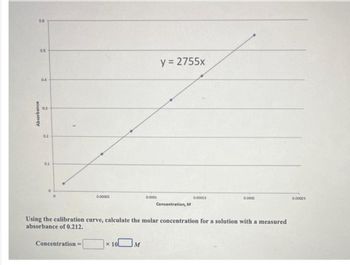
Transcribed Image Text:0.6
Absorbance
0.5
04
3
0.2
0.1
0
O
0.00005
Concentration=
x 10
0.0001
M
y = 2755x
Concentration, M
Using the calibration curve, calculate the molar concentration for a solution with a measured
absorbance of 0.212.
0.00015
0.0002
0.00025
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Time Left:0:55:00 A student completes the experiment The Universal Gas Constant and obtains the following data for one trial. mass of magnesium (g): Initial gas volume (ml): Final Volume (mL): Temperature (°C): Atmospheric pressure (inHg): Ah (cm of water): F3 80 모 Calculate the partial pressure of hydrogen, PH2, for this trial. Give your answer in torr (mmHg). 1 in Hg = 25.4 mmHg 1 cm water = 0.735559 mmHg 1 mol Mg = 24.305 g Mg TABLE D-4 TEMP 1_DEG₁_C_1 1 3 I 1 1 Q F4 0 1 = 2 3 4 5 6 7 8 10 11 12 13 14 0.0 0.1 0.0326 0.00 35.30 21.3 30.21 16 17.13 F5 6.100 | 6.143 1 6.544 1 6.589 0.2 1 VAPOR PRESSURE OF HATER 0-30 DEG. C IN MM HG 1 4.579 4.612 4.646 | 4.924 | 4.959 4.995 15.291 4.660 4.714 5.0315.068 5.329 5.367 5.406 5.445 4.7484.783 4.818 5.104 | 5.141 | 5.178 5.484 5.523 5.563 5.888 5.930 6.230 16.274 6.318 5.363 15.683 15.723 | 5.764 | 5.8051 5.146 6.186 6.636 5.972 1 6.0141 6.057 1 6.407 1 6.453 6.498 1 6.82 | 6.729 6.776 6.823 | 6.871 | 6.919 | 6.967 1 T T 1 I T I 1 17.0167,064…arrow_forward(Q33) What is the root mean square speed (URMS) of fluorine gas at -36.7 °C?arrow_forwardIf 4.00 L of water vapor at 50.2 °C and 0.121 atm reacts with excess iron, how many grams of iron(III) oxide will be produced? 2 Fe(s) + 3H2O(g) → Fe2O3 (s) + 3H2(g)arrow_forward
- Hi! I am unsure of how to solve this problem. Thank you!arrow_forwardOzone molecules in the stratosphere absorb much of the harmful radiation from the sun. How many ozone molecules are present in 1.00 L of air under the stratospheric ozone conditions of 249 K temperature and 1.51 x 10 -3 atm pressure? Be sure your answer has the correct number of significant digits. molecules X S ∞o E Olo Ararrow_forwardChemistry helpppp.arrow_forward
- Dinitrogen monoxide causes depletion of ozone in the in the stratosphere. Different sources of N20 have different ratios 14N and 15N. a. State one analytical technique that could be used to determine the ratio of 14N:15N b. A sample of gas was enriched to contain 2% by mass of 15N with the remainder being 14N. Calculate the relative molecular mass resulting of N2O.arrow_forwardPlease answer the question and show your work. 6a. A helium balloon was partially filled with 8,000 ft3 (cubic feet or ft3, is a unit of volume) of helium gas when the atmospheric pressure was 0.98 atm and the temperature was 23 °C. The balloon rose to an altitude where the atmospheric pressure was 400 mm Hg and the temperature was 5.3 °C. What volume in ft3 did the helium gas occupy at this altitude?arrow_forwardKk.19. Sulfur dioxide (SO2) is a by-product of burning coal and contributes to the formation of acid rain. The mole fraction of SO2 in air is 2 ppm. How many molecules of SO2 are present in 1.50 x 10^6 L of air when the barometric(total) pressure is 1.1 atm and the temperature is 32 °C?arrow_forward
- After the following reaction is balanced: C₆H₁₂O₆ + O₂ →CO₂ + H₂O. What are the stoichiometric coefficients of C₆H₁₂O₆, O₂, CO₂ and H₂O, respectively.arrow_forward#69arrow_forwardA quantity of helium gas is originally held under a pressure of 145 kPa at 18°C. If the volume of the container is tripled, and the temperature is increased to 30°C, what is the resulting pressure in kPa? (Hint, can you make up a volume?) H 322181 Li Na Mg Rb Be Sr Cs Ba Fr 22 Ca Sc Ti Ra Raden Lat 94.5 kPa 46.4 kPa 378.3 kPa 453.9 kPa 50.3 kPa MD 45 83-103 482.2 kPa Zr Hf Atvice Ac Th Series 23 VO Vieta VID 24 2 Ta W Tete 104 105 Rf Db Sg Cr Mn Periodic Table of the Elements VID 75 41. 34445 Nb Mo Tc Ru Rh Pd Ag Cd Abaline Tra Saber 26 27 28 29 30 Fe Co Ni Cu Zn Re Os Symbol S 13 13 FIA B Nawww Al Ga Halagan In La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho D IVA 70 Pt Au Hg Tl Pb B Mar 102108, 109 114 Bh Hs Mt Ds Rg Cn Uut "FI 590 U Np Pu Am Cm Bk Cf Es Sn Sb 14 15 16 17 18 Si CI Ar La 16 3514. Ge As Se Br Kr By 17 VIA 18 VELA He Hek 4000 Ne 62 63 64 SA Te I Xe 16 At Rn Fate Bi Po Uup "Lv Uus Uuo Er Tm Yb Lu C Henan 100 100 Fm Md No Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY