
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
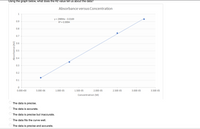
Transcribed Image Text:Using the graph below, what does the R2 value tell us about the data?
Absorbance versus Concentration
1
y = 29894x - 0.0169
R² = 0.9994
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.00E+00
5.00E-06
1.00E-05
1.50E-05
2.00E-05
2.50E-05
3.00E-05
3.50E-05
Concentration (M)
The data is precise.
The data is accurate.
The data is precise but inaccurate.
The data fits the curve well.
The data is precise and accurate.
Absorbance (AU)
O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The wavelength of maximum absorbance of methyl red is closest to ___. Question 20 options: 570 nm 617 nm 431 nmarrow_forwardLet's pretend your little cousin walked in on you as you were performing your experiment. They took your dyed liquid and made their own sample by diluting it with water just as you did. However you did not see how much water they added. Assume the graph below represents your measured results. Now lets say you take one more image using your little cousin's sample of unknown concentration, and you calculate the absorption value to be 0.34. What is the approximate concentration of the solution that your little cousin mixed?arrow_forward5. A sample of a red dye and a sample of a blue dye both have the same concentration. Their Absorbance is measured using the same spectrophotometer. At a particular wavelength, will both samples give the same Absorbance reading? Why or why not?arrow_forward
- What is the molarity of p-nitroaniline in a solution if the absorbance is 0.504 assuming a path length of 1.00 cm?arrow_forwardYou want to figure out the caffeine concentration in a coffee you bought. You make four caffeine standard solutions of increasing concentration and fit the data. Your line fit has the following equation: y = 0.053(AU/mM) X + 0.0012AU You test your coffee sample and measure an absorbance of 0.82 AU. What is the concentration ○ 0.0042 mM ○ 0.0042 M ○ 15 mM ○ 0.043 mM ○ 15 M 0.043 Marrow_forwardheres the data TZ # Concentration Absorbance at 430 nmnm 1 33.6480 0.931 2 25.2360 0.757 3 16.8420 0.210 4 8.41200 0.137 5 4.20600 0.122arrow_forward
- 3. What is the purpose of using blank before measuring the absorbance of a substance?arrow_forwardGiven the absorbance spectrum shown above for substance A in solution, what wavelength would you choose for absorbance/concentration studies for solutions of A? A 470 nm B 520 nm C 585 nm D 600 nm E 650 nmarrow_forwardIf the concentration of a food coloring solution is increased by a factor of 3, how will the absorbance change?arrow_forward
- Given this data, if a CV solution has an absorbance of 1.125, what is [CV]? [CV] / M A 1.0 × 10–6 M 0.250 3.0 × 10–6 M 0.750 Question 12 options: 4.5 × 10–6 M 5.0 × 10–6 M 5.5 × 10–6 Marrow_forwardSolve for the entire table 2 show all work typed. Double-check your answers.arrow_forwardExamine the given Beer's law standard curve for an unknown dye measured in a 1.0 cm cuvette. Absorbance of Unknown Dye at 542 nm 0.5 E 0.45 0.4 0.35 0.3 0.25 0.2 y = 15200x - 0.018. 6 0.15 * 0.1 5.00E-06 1.00E-05 1.50E-05 2.00E-05 2.50E-05 3.00E-05 3.50E-O5 Concentration (M) What is the concentration (in M) of a sample of the unknown dye with an absorbance of 0.16 at 542 nm? Type answer: ВАCK Question 8 of 8 REVIEW A «tv A O Absorbance at 542 nmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY